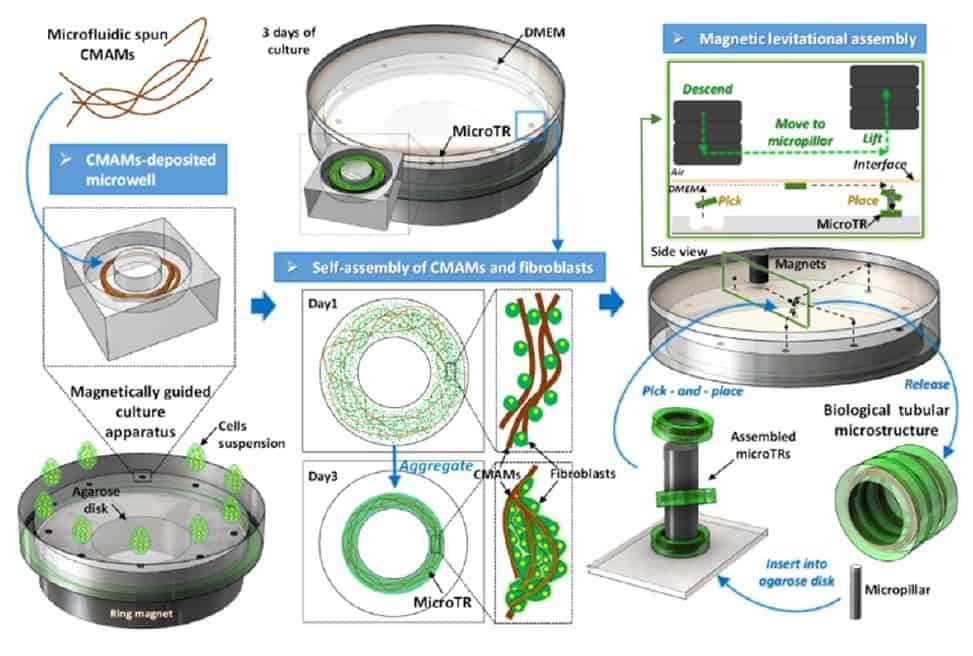
Tao Sun and colleagues from Beijing Institute of Technology have described a novel method to incorporate synthetic microfibres containing magnetic beads into self-assembled tissue micro-rings. Magnetic force, coupled with a smart usage of surface tension, enabled the adhesion and proliferation of fibroblasts on the micro-rings, as well as subsequent stacking of the rings into tubular structures (Biofabrication 10.1088/1758-5090/ab1ee5).
Finding tissue donors can be a limiting factor in the treatment of several diseases. For this reason, tissue engineering has become a booming field in the last decade, with scientists striving in their quest to replicate functional tissues in vitro. Many fundamental structures in the human body display a tubular shape (the trachea, oesophagus and blood vessels, for example) that researchers try to replicate.
Current trends in tissue engineering include the use of hydrogels, which have many advantages but come with a price: inhomogeneous cell distribution, as well as poor mechanical properties and limited nutrient diffusion. Alternative approaches to the direct delivery of cell-supplemented hydrogels to the injury site are already established. Among these is bottom-up structural assembly of biological tubes using tissue rings with incorporated microscaffolds. However, few microscaffolds are currently available as platforms. Therefore, Sun and colleagues examined whether tissue rings with incorporated microscaffolds could be created from microfluidic spun hydrogel microfibres.
In this study, the researchers incorporated magnetic particles in crosslinked calcium-alginate hydrogel fibres, with diameters optimized to provide the best support for cell growth and diffusion. To better hook cells, they coated the microspun fibres with poly-L-lysine residues bound to a fibronectin network.
To align the fibres in a parallel direction, the researchers exploited the surface tension of the fibronectin solution while transferring the rings in a culture dish placed onto a magnet before seeding fibroblasts on them. Thanks to the incorporated magnetic beads, they were then able to avoid floating of the rings, allowing for fibroblast seeding. Cell seeding was successful, with fibroblasts eventually spreading uniformly on the fibres, forming multiple layers and displaying good morphology and viability.
After three days of incubation, the rings self-assembled: the fibres guided the growth of fibroblasts, creating connections between themselves and the microgel, which resulted in the progressive shrinkage of the annular structures. The researchers then used magnetic force to serially assemble the micro-rings on top of each other around an inner pillar. This formed a cylinder that was cultured for five more days to let the cells seal the rings together. The result closely resembled the structure of a blood vessel.
Having provided this important proof-of-concept for bottom-up tissue engineering, the authors plan some important modifications to this platform in their forthcoming studies. These include addressing the observed formation of cell aggregates (possibly due to limited fibronectin coating), as well as the limited guidance of cell orientation. They envision that the substitution of alginate hydrogel microfibres with methacrylamide-modified gelatin (GelMA) microfibres will do the job.



