Paweł Moskal from Jagiellonian University talks to Tami Freeman about positronium imaging with the unique J-PET scanner and his team’s work in applying quantum entanglement to diagnostics
Positron emission tomography (PET) is a diagnostic imaging technique that uses an injected radioactive tracer to detect early signs of cancer, brain disorders or other diseases. At Jagiellonian University in Poland, a research team headed up by Paweł Moskal is developing a totally new type of PET scanner. The Jagiellonian PET (J-PET) can image the properties of positronium, a positron–electron bound state produced during PET scans, offering potential to increase the specificity of PET diagnoses.
The researchers have now recorded the first ever in vivo positronium image of the human brain. They also used the J-PET to show that annihilation photons generated during PET scans are not completely quantum entangled, opening up the possibility of using the degree of quantum entanglement as a diagnostic indicator. Moskal tells Physics World’s Tami Freeman about these latest breakthroughs and the team’s ongoing project to build the world’s first whole-body quantum PET scanner.
Can you describe how conventional PET images are generated?
PET is based on the annihilation of a positron with an electron to create two photons. The patient is administered a radiopharmaceutical labelled with a positron-emitting radionuclide (for example, fluoro-deoxy-glucose (FDG) labelled with 18F), which localizes in targeted tissues. The 18F emits positrons inside the body, which annihilate with electrons from the body, and the resulting annihilation photons are registered by the PET scanner.
By measuring the locations and times of the photons’ interactions in the scanner, we can reconstruct the density distribution of annihilation points in the body. With 18F-FDG, this image correlates with the density distribution of glucose, which in turn, indicates the rate of glucose metabolism. Thus the PET scanner delivers an image of the radiopharmaceutical’s metabolic rate in the body.
Such an image enables physicians to identify tissues with abnormal metabolism, such as cancers that metabolize glucose up to 10 times more intensively than healthy tissues. Therefore, PET scanners can provide information about alterations in cell function, even before cancer may be visible in anatomical images recorded using CT or MRI.
During annihilation, a short-lived atom called positronium can form. What’s the rationale for imaging this positronium?
It’s amazing that in tissue, positron–electron annihilation proceeds via the formation of positronium in about 40% of cases. Positronium, a bound state of matter and antimatter (an electron and a positron), is short lived because it can undergo self-annihilation into photons. In tissue, however, it can decay via additional processes that further shorten its lifetime. For example, its positron may annihilate by “picking off” an electron from a surrounding atom, or it may convert from the long-lived state (ortho-positronium) to the short-lived state (para-positronium) through interaction with oxygen molecules.
In tissue, therefore, positronium lifetime is an indicator of the intra- and inter-molecular structure and the concentration of oxygen molecules. Both molecular composition and the degree of oxygen concentration differ between healthy and cancerous tissues, with hypoxia (a deficit in tissue oxygenation) a major feature of solid tumours that’s related to the development of metastases and treatment resistance.
As such, imaging positronium lifetime can help in early disease recognition at the stage of molecular alterations. It can also improve diagnosis and the proper choice of anti-cancer therapy. In the case of brain diagnostics, positronium imaging may become an early diagnostic indicator for neurodegenerative disorders such as dementia, Alzheimer’s disease and Parkinson’s disease.
So how does the J-PET detect positronium?
To reconstruct the positronium lifetime we use a radionuclide (44Sc, 82Rb or 124I, for example) that, after emitting a positron, promptly (within a few picoseconds) emits an additional gamma photon. This “prompt gamma” can be used to measure the exact time that the positron was emitted into the tissue and formed positronium.
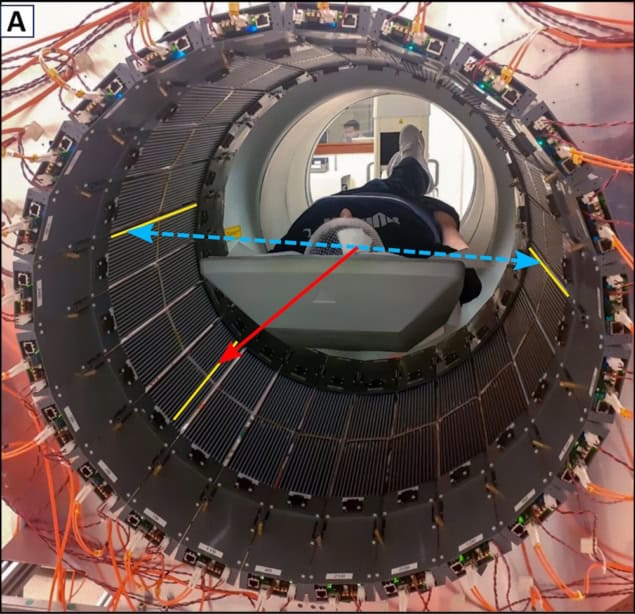
Current PET scanners are designed to register only two annihilation photons, which makes them incapable of determining positronium lifetime. The J-PET is the first multiphoton PET scanner designed for simultaneous registration of any number of photons.
The registration of annihilation photons enables us to reconstruct the time and location of the positronium decay, while registration of the prompt gamma provides the time of its formation. The positronium lifetime is then calculated as the time difference between annihilation and prompt gamma emission.
Can you describe how your team recorded the first in vivo positronium image?
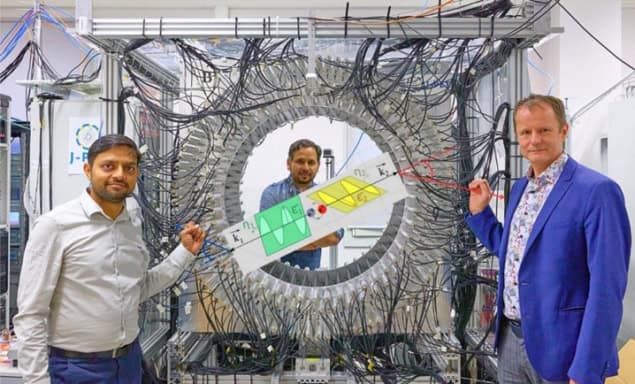
Last year we presented the world’s first in vivo images of positronium lifetime in a human, reported in Science Advances. For this, we designed and constructed a modular, lightweight and portable J-PET tomograph, consisting of 24 independent detection modules, each weighing only 2 kg. The device uses a multiphoton data acquisition system, invented by us, to simultaneously register prompt gamma and annihilation photons – the first PET scanner in the world to achieve this.
The research was performed at the Medical University of Warsaw, with studies conducted following routine procedures so as not to interfere with routine diagnostics and therapy. If a patient agreed to stay longer on the platform, we had about 10 minutes to install the J-PET tomograph around them and collect data.
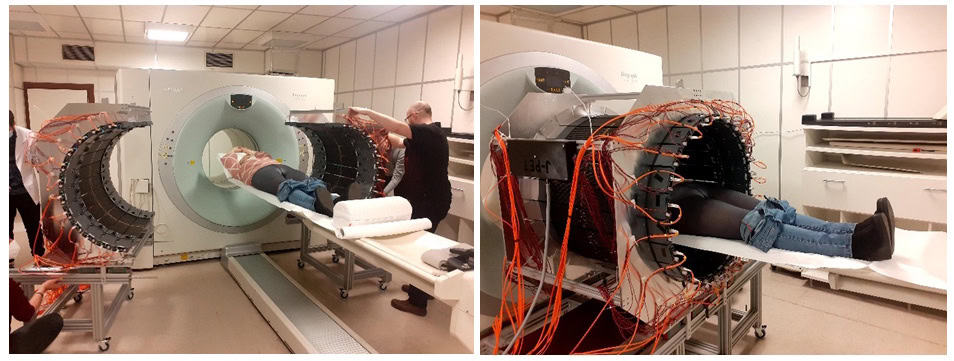
The first patient was a 45-year-old man with glioblastoma (an aggressive brain tumour) undergoing alpha-particle radiotherapy. The primary aim of his therapy was to destroy the tumour using alpha particles emitted by the radionuclide 225Ac. The positronium imaging was made possible by the concurrent theranostic application of the radionuclide 68Ga to monitor the site of cancer lesions using a PET scanner.
The patient was administered a simultaneous intra-tumoural injection of the alpha-particle-emitting radiopharmaceutical (225Ac-DOTA-SP) for therapy and the positron emitting pharmaceutical (68Ga-DOTA-SP) for diagnosis. In about 1% of cases, after emitting a positron that annihilates with an electron, 68Ga also emits a prompt gamma ray.
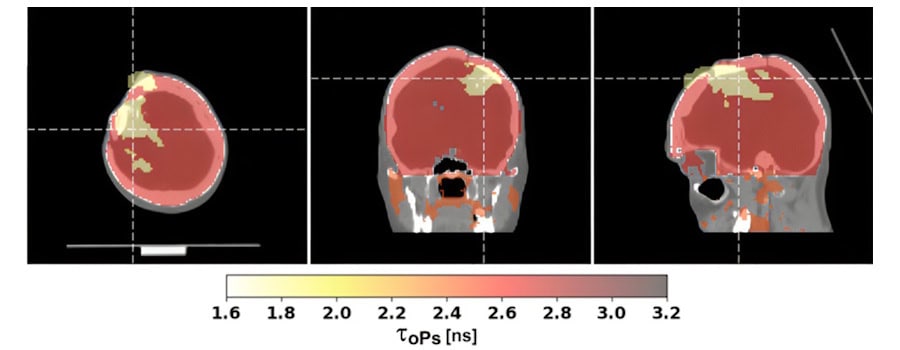
We determined the annihilation location by measuring the time and position of interaction of the annihilation photons in the scanner. For each image voxel, we also determined a lifetime spectrum as the distribution of differences between the time of annihilation and the time of prompt gamma emission.
Our study found that positronium lifetimes in glioblastoma cells are shorter than in salivary glands and healthy brain tissues. We showed for the first time that the mean lifetime of ortho-positronium in a glioma (1.77±0.58 ns) is shorter than in healthy brain tissue (2.72±0.72 ns). This finding demonstrates that positronium imaging could be used for in vivo diagnosis to differentiate between healthy and cancerous tissues.
You recently demonstrated that J-PET can detect quantum entanglement of annihilation photons, how could this impact cancer diagnostics?
For this study, reported earlier this year in Science Advances, we used the laboratory prototype of the J-PET scanner (as employed previously for the first ex vivo positronium imaging). The crucial result was the first ever observation that photons from electron–positron annihilation in matter are not completely quantum entangled. Our study is pioneering in revealing a clear dependence of the degree of photon entanglement on the material in which the annihilation occurs.
These results are totally new compared with all previous investigations of photons from positron–electron annihilations. Up to this point, all experiments had focused on showing that this entanglement is maximal, and for that purpose, were performed in metals. None of the previous studies mentioned or even hypothesized a possible material dependence.
If the degree of quantum entanglement of annihilation photons depends on the material, it may also differ according to tissue type or the degree of hypoxia. This is a hypothesis that we will test in future studies. I recently received an ERC Advanced Grant, entitled “Can tissue oxidation be sensed by positronium?”, to investigate whether the degree of oxidation in tissue can be sensed by the degree of quantum entanglement of photons originating from positron annihilation.
What causes annihilation photons to be entangled (or not)?
Quantum entanglement is a fascinating phenomenon that cannot be explained by our classical perception of the world. Entangled photons behave as if one instantly knows what is happening with the other, regardless of how far apart they are, so they propagate in space as a single object.
Annihilation photons are entangled if they originate from a pure quantum state. A state is “pure” if we know everything that can be known about it. For example, if the photons originate from the ground state of para-positronium (a pure state), then we expect them to be maximally entangled.
However, if electron–positron annihilation occurs in a mixed state (a statistical mixture of different pure states) where we have incomplete information, then the resulting photons will not be maximally entangled. In our case, this could be the annihilation of a positron from positronium with electrons from the patient’s body. Because these electrons can have different angular momenta with respect to the positron, the annihilation generally occurs from a mixed state.
You have also measured the polarization of the annihilation photons; how is this information used?
In current PET scanners, images are reconstructed based on the position and time of interaction of annihilation photons within the scanner. However, annihilation photons also carry information about their polarization.
Theoretically, annihilation photons are quantum entangled in polarization and exhibit non-local correlations. In the case of electron–positron annihilation into two photons, this means that the amplitude of the distribution of the relative angle between their polarization planes is larger when they are quantum entangled than when they propagate in space as independent objects.
State-of-the-art PET scanners, however, cannot access polarization information. Annihilation photons have energy in the mega-electronvolt range and their polarization cannot be determined using established optical methods, which are designed for optical photons in the electronvolt range. Because these energetic annihilation photons interact with single electrons, their polarization can only be sensed via Compton scattering.
The angular distribution of photons scattered by electrons is not isotropic with respect to the polarization direction. Instead, scattering is most likely to occur in a plane perpendicular to the polarization plane of the photon before scattering. Thus, by determining the scattering plane (containing the primary and scattered photon), one can estimate the direction of polarization as being perpendicular to that plane. Therefore, to practically determine the polarization plane of the photon, you need to know its directions of flight both before and after Compton scattering in the material.
In plastic scintillators, annihilation photons primarily interact via the Compton effect. As the J-PET is built from plastic scintillators, it’s ideally suited to provide information about the photons’ polarization, which can be determined by registering both the annihilation photon and the scattered photon and then reconstructing the scattering plane.
Using the J-PET scanner, we determined the distribution of the relative angle between the polarization planes of photons from positron–electron annihilation in a porous polymer. The amplitude of the observed distribution is smaller than predicted for maximally quantum-entangled two-photon states, but larger than expected for separable photons.
This result can be explained by assuming that photons from pick-off annihilation are not entangled, while photons from direct and para-positronium annihilations are maximally entangled. Our finding indicates that the degree of entanglement depends on the annihilation mechanism in matter, opening avenues for exploring polarization correlations in PET as a diagnostic indicator.
What further developments are planned for the J-PET scanner?
When creating the J-PET technology, we started with a two-strip prototype, then a 24-strip prototype in 2014, followed by a full-scale 192-strip prototype in 2016. In 2021 we completed the construction of a lightweight (60 kg) J-PET version that is both modular and portable, and which we used to demonstrate the first clinical images.
The next step is the construction of the total-body quantum J-PET scanner. We are now at the stage of collecting all the elements of this scanner and expect to complete construction in 2028. The scanner will be installed at the Center for Theranostics, established by myself and Ewa Stępień, medical head of the J-PET team, at Jagiellonian University.
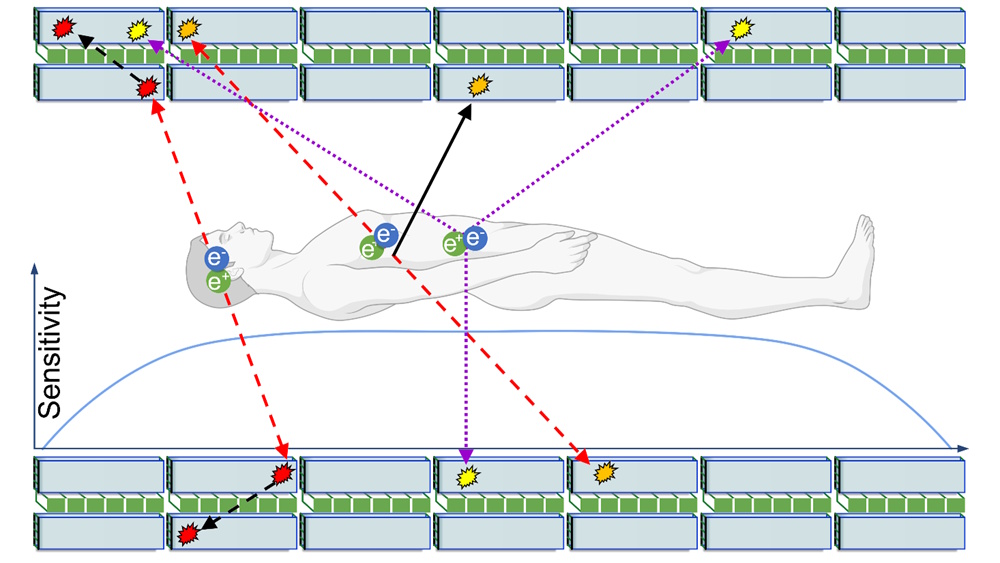
Total-body PET provides the ability to image the metabolism of all tissues in the body at the same time. Additionally, due to the high sensitivity of total-body PET scanners, it is possible to perform dynamic imaging – essentially, creating a movie of how the radiopharmaceutical distributes throughout the body over time.
The total-body J-PET will also be able to register the pharmacokinetics of drugs administered to a patient. However, its true distinction is that it will be the world’s first quantum PET scanner with the ability to image the degree of quantum entanglement of annihilation photons throughout the patient’s body. Additionally, it will be the world’s first total-body multiphoton PET, enabling simultaneous positronium imaging in the entire human body.
How do you see the J-PET’s clinical applications evolving in the future?
We have already performed the first clinical imaging using J-PET at the Medical University of Warsaw and the University Hospital in Kraków. The studies included the diagnosis of patients with neuroendocrine, prostate and glioblastoma tumours. The data collected at these hospitals were used to reconstruct standard PET images as well as positronium lifetime images.
First positronium image recorded during a PET scan
Next, we plan to conduct positronium imaging of phantoms and humans with various radionuclides to explore its clinical applications as a biomarker for tissue pathology and hypoxia. We also intend to explore the J-PET’s multiphoton capabilities for simultaneous double-tracer imaging, as well as study the degree of quantum entanglement as a function of the annihilation mechanism.
Finally, we plan to explore the possibilities of applying quantum entanglement to diagnostics, and we look forward to performing total-body positronium and quantum entanglement imaging with the total-body J-PET in the Center for Theranostics.
- Paweł Moskal is a panellist in the forthcoming Physics World Live event on 25 September 2025. The event, which also features Miles Padgett from the University of Glasgow and Matt Brookes from the University of Nottingham, will examine how medical physics can make the most of the burgeoning field of quantum science. You can sign up free here.




