A novel light-focusing technique could enable diagnostic and therapeutic applications, as Jude Dineley explains
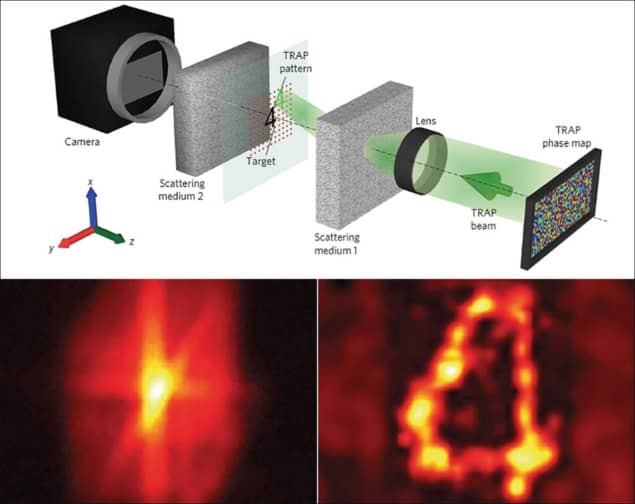
A non-invasive, non-contact technique that focuses light on moving structures in scattering materials has been developed by researchers in the US. Dubbed time-reversed adapted-perturbation (TRAP) optical focusing, the technique can be used in soft tissue and has a range of potential medical and biological applications (Nature Photonics 8 931).
In medicine, the ability to focus light at depth in tissue – a strongly scattering medium – is valuable in techniques including photoacoustic imaging and photoablation therapies. Existing strategies use a “guide star” – a reference source that characterizes scatter in the medium. The problem is that physical versions, such as implanted fluorescence beads, are invasive, while “virtual” guide stars, which use focused ultrasound, have to be placed in direct contact with skin, limiting clinical applications. TRAP, in contrast, seeks out moving structures and uses them instead.
“The original motivation for inventing TRAP was to develop a technology that can focus inside tissue non-invasively and without physical contact,” says lead author Cheng Ma of the Optical Imaging Laboratory at Washington University in St Louis. However, the researchers soon realized that TRAP can enable several diagnostic and therapeutic applications that are not possible with conventional focusing.
Therapeutic and diagnostic applications
For example, by detecting and focusing light on flowing blood, the technique could be used in the targeted ablation of the enlarged blood vessels of port-wine stains (red or purple marks on the skin caused by abnormal blood-vessel growth). “TRAP can guide light to bypass the stationary portion of the tissue, the extravascular space to be protected, and focus only onto the targeted vessels to be treated,” explains lab director Lihong Wang.
The detection of flowing blood could also benefit photoacoustic imaging techniques such as photoacoustic computed tomography (PACT), which exploits the absorption of light in blood vessels to build functional images of how much oxygen is in the blood. By focusing light only on the vessels, the photoacoustic signal is increased, which increases image contrast and the depth at which vessels can be imaged.
TRAP uses a pulsed laser to illuminate a sample of a scattering medium, with the transmitted 2D scatter field recorded by an interferometer downstream. After a short delay, in which the intended focusing target has moved within the medium, the process is repeated. By subtracting one scatter field from the other, the old and new locations of the target are revealed, while eliminating signals from the stationary medium.
The distribution is converted to a map that describes the equivalent modulation in phase between the two images. The map is displayed downstream of the medium on a liquid-crystal spatial light modulator (SLM) – a phase conjugate mirror. A beam that is then fired at the mirror “reads” the map and gets phase-conjugated upon reflection, which time reverses the signal, focusing it onto the moving target. Repeated scatter snapshots allow the focused light to track the target.
In an experimental assessment, the researchers used TRAP to detect and focus light on blood from a cow pumped through a tube embedded between two pieces of chicken-breast tissue. It focused light in the blood to three times the intensity in the surrounding chicken. In a second investigation, TRAP was successfully used to detect a chromium marker and focus light on it after it was moved from outside to inside an illuminated region.
TRAP is far from perfect though. The researchers showed that its focusing ability deteriorates if the scattering medium moves. They are currently trying to minimize the effect with improvements that will speed up the technique. They also plan to improve TRAP’s focusing power.
In its current form, these two issues limit TRAP’s use to experiments in vitro and on excised tissue samples. “We are pushing hard to address these issues to make the technique useful for in vivo clinical uses,” says Ma, adding that the group also plans to explore a reflective implementation of TRAP, which would have a wider range of clinical uses.



