A new treatment planning system (TPS) for proton therapy has been shown to accurately predict delivered dose by modelling the beam as a combination of three Gaussian distributions. Researchers in Japan tested the system using an ionization chamber and radiochromic film in a simple, commercially available phantom. The results demonstrate a reliable and convenient method of dose verification that could be adopted widely by proton therapy providers (J. Appl. Clin. Med. Phys. 10.1002/acm2.12535).
Because the depth at which a proton beam is halted by tissue depends on its initial energy, intensity-modulated proton therapy (IMPT) allows the radiation field to conform closely to the 3D shape of the tumour while sparing surrounding tissue. This makes IMPT the method of choice for intricately shaped tumours in complex physiological settings. The narrow margins in these situations mean that a robust quality assurance procedure is needed so that clinicians can be confident that the planned dose is the one that is delivered to the patient.
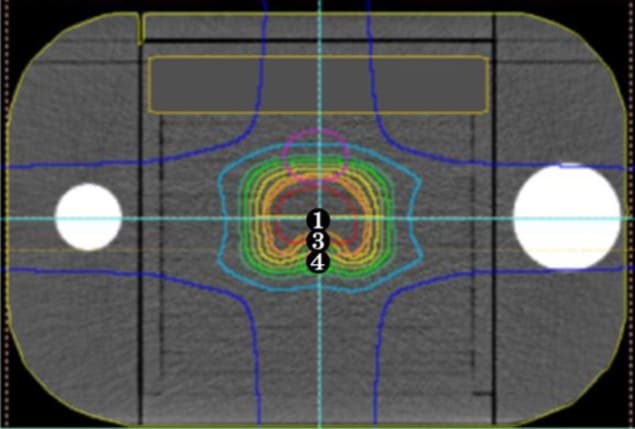
To establish such a procedure for pencil-beam scanning proton therapy, Keisuke Yasui and colleagues, at Fujita Health University and Nagoya Proton Therapy Centre, simulated treatments for prostate and head-and-neck cancers using a simple commercial phantom that broadly replicates the form of a human patient. The researchers placed an ionization chamber at various points within the phantom to measure the absolute dose, and inserted radiation-sensitive film at three different depths to find the relative dose distribution.
Although the phantom is constructed using materials that approximate the radiation-absorbing properties of human tissue, the equivalence is not quite perfect. This means that standard calculations used to predict how the proton beam is stopped by tissue could not be applied to the phantom directly. To account for this, Yasui and colleagues derived a correction factor that converts measured radiodensity — in Hounsfield units — to phantom-specific relative stopping power. They also considered errors in the predicted dose that arise due to uncertainty in the measurement point of the ionization chamber, and in the point around which the treatment gantry rotates.
Triple Gaussian model
Key to the reliability of the group’s results is their use of a new TPS that better models the dose profile of the proton beam. Whereas traditional approaches represent the dose cross-section as a sum of two Gaussian distributions, previous research (Med. Phys. 10.1118/1.4942386) has shown that, under certain conditions, a combination of three distributions more accurately captures the effect of secondary particles at the beam’s edge.
As the TPS and phantom are both commercially available, any clinic that uses IMPT based on pencil-beam scanning can use the procedure and the team’s phantom-specific correction table to verify their treatment plans. As long as absolute dose measurements are taken for each beam angle — to mitigate uncertainty related to measurement points and gantry rotation — the method provides an accurate, reproducible basis for quality assurance. “Our motivation was to realize equal access to high-quality spot-scanning proton therapy in Japan and all over the globe,” says Yasui.
3D printing enables gel dosimetry in complex phantoms
Next, Yasui and colleagues intend to investigate the value of using radiophotoluminescent glass dosimeters in place of ionization chambers. When such dosimeters absorb ionizing radiation such as protons, they develop stable luminescent centres that can later be excited using ultraviolet light. These dosimeters are attractive for their reproducibility, dose linearity and angular independence, and have already been used successfully in a multi-institution photon-beam dose audit in Japan.
In the future, the researchers also hope to use a 3D printing method to create a dosimeter that measures 3D dose distribution.