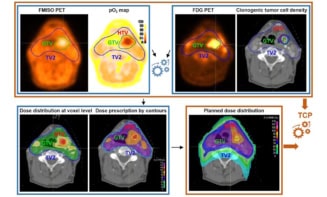
Tumour hypoxia, defined as low levels of oxygen partial pressure (pO2), can cause resistance to radiotherapy. The ability to characterize tumour hypoxia could help to optimize radiation treatments. But there’s currently a lack of methods to monitor tumour oxygenation noninvasively or without averaging across the entire lesion.
Recently, a team headed up at Dartmouth-Hitchcock’s Norris Cotton Cancer Center developed a new imaging approach that combines phosphorescence quenching with excitation by the Cherenkov light generated within irradiated tissues during radiotherapy. They have now used this approach – called Cherenkov excited luminescence imaging (CELI) – to non-invasively image oxygen distribution in mouse tumours during radiation delivery (Nature Commun. 10.1038/s41467-020-14415-9).
For their study, Brian Pogue and his team employed time-resolved CELI with the phosphorescent probe Oxyphor PtG4. They irradiated mouse tumours with pulsed megavoltage X-ray irradiation, generating Cherenkov light that served as an internal source to excite the probe’s phosphorescence. They employed a time-gated camera to capture emission at different delay times after each radiation pulse, and then used these images to determine phosphorescence lifetimes and obtain tissue pO2 values.
“The imaging is all done without any additional radiation, simply by using a camera to monitor the emissions during radiotherapy,” explains Pogue. “We have a unique set of time-gated cameras in our radiation therapy department that were designed for Cherenkov-based radiation dosimetry, but we have used them for this additional purpose of monitoring oxygen in the tumours under treatment.”
Imaging of mice injected with PtG4 revealed that the drug stays in the tumour for at least five days. This provides the opportunity to employ CELI over multiple radiation fractions using a single PtG4 injection, allowing tracking of oxygen dynamics in tumours throughout the treatment course.
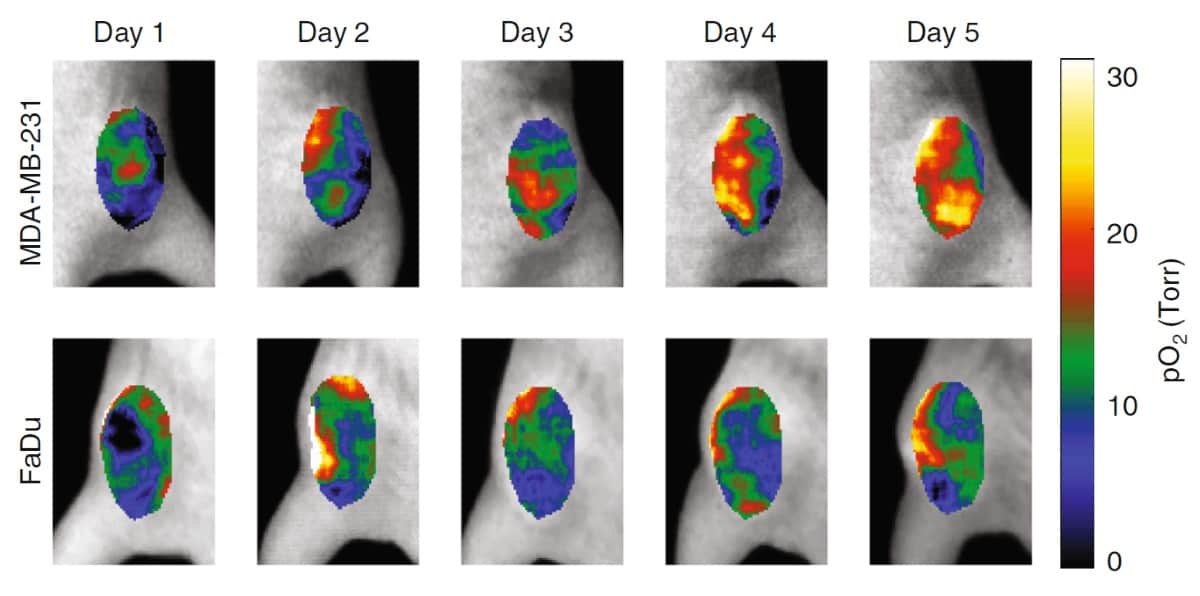
To test the approach, the researchers performed CELI on six mice with subcutaneous tumours during five days of hypofractioned radiation therapy (5 Gy/fraction), following a single intravascular injection of PtG4. The tumours were either radiosensitive MDA-MB-231 breast cancer or radioresistant FaDu head-and-neck cancer xenografts.
CELI performed during each fraction showed that pO2 distributions in both tumours were highly heterogeneous. The hypoxic regions decreased in size from one fraction to the next, with a far more pronounced decrease in the MDA-MB-231 tumours. The median pO2 values in the radiosensitive tumours increased markedly as treatment progressed, while no obvious changes were seen in the radioresistant tumours. Tumour response was delayed relative to the response of the local pO2, with MDA-MB-231 tumours starting to shrink five days after the start of therapy and FaDu tumours only shrinking on day nine.
“Following two tumour lines, one which is known to be responsive to radiation and one which is known to be resistant, we could see differences in the oxygenation of the tumour which are reflective of their differences in response,” says Pogue.
Clinical goals
The excitation source in CELI is the Cherenkov light generated along the treatment beam path, meaning that probe excitation occurs at all depths where radiation dose is deposited. Detection of the phosphorescence, however, is limited by tissue absorption and scattering. The researchers estimate that the current maximum imaging depth is about 2 cm.
They suggest that this depth can be increased by employing brighter phosphorescent probes or using cameras with sensitivity optimized for the 750–850 nm phosphorescence spectral range. This could extend the potential applications of CELI to include imaging of near-surface tumours, intracavity measurements using catheter-based cameras, and pre-clinical assessments of oxygenation during therapy in lab animals.
Optical imaging provides quality assurance for small radiotherapy beams
The researchers conclude that, compared with current clinical pO2 measurement modalities, CELI is capable of significantly higher spatial resolution and allows image acquisition simultaneously with multiple fractions of radiotherapy. They note that the method can be easily added to clinical protocols, enabling evaluation of tumour pO2 at the time of the radiation dose delivery – a long-sought goal in tumour therapy.
The team is now characterizing how small a region they can track the oxygenation from, and how fast they can take measurements. “Our goal is to produce oxygen images at video rate, with a spatial resolution that allows us to see radiobiologically relevant hypoxia nodules in tumours of humans,” says Pogue.