Physicists have created a new form of electron microscopy that can make "movies" of atoms as they undergo ultra-rapid chemical or structural transitions. Ahmed Zewail and colleagues at the California Institute of Technology in the US have used coincident electron and laser pulses to follow vanadium and oxygen atoms as they rearranged themselves on a vanadium oxide surface over the course of several picoseconds. The researchers say that the technique could also be used to study a wide range of ultrafast biological and physical phenomena. (Proc. Natl. Acad. Sci. 103 18427)).
Electron microscopes have better resolution than optical microscopes because high-energy electrons have a much shorter wavelength than light. The resolution can be further improved by using coherent electron wavepackets, which can contain as few as one electron. The wavelengths of these packets are much smaller than the space between individual atoms and can be brought to a very sharp focus, allowing objects to be imaged with atomic-scale resolution. The packets are of extremely short duration and this can be exploited to take “snapshots” of atoms as they undergo structural or chemical transitions.
In 2005, Zewail and colleagues used coherent electron packets to take single snapshots of a number of materials and biological samples. Now the researchers have further refined their technique to take a time sequence of images that allowed them to watch vanadium and oxygen atoms rearrange themselves in a process that can take as little as 100 femtoseconds ( 10-13 seconds).
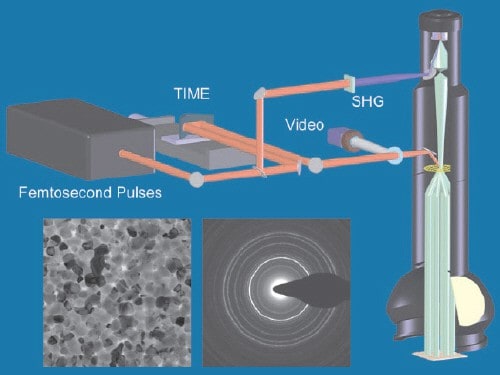
The timing sequence is generated by femtosecond laser pulses as illustrated in the figure “Ultrafast microscope”. Each pulse is split into two pulses – one is used by the microscope to create the electron pulse and the other is used to heat the sample. According to Zewail, the crucial and most difficult part of the technique is coordinating the arrivals of the laser and electron pulses at the sample with an accuracy of just a few femtoseconds. This is particularly difficult because the laser pulse travels at the speed of light, while the electron pulse lags behind at about two thirds the speed of light.
The coincident laser pulse is used to heat the sample and drive a transition from a low-temperature crystal structure to a high-temperature structure. By changing the delay between the laser and electron pulses in regular time steps, the researchers were able to take snapshots of the atoms at different sample temperatures.
Zewail and colleagues found that vanadium oxide undergoes a “first-order” phase transition from a low-temperature “monoclinic” phase to a high-temperature tetragonal “rutile” phase at around 67°C. This result is a breakthrough in itself because the precise nature of this transition has been a mystery since the material was discovered almost a century ago.
The team now plans to try their technique on other materials; “the scope is very wide from semiconductors and metals to organics and biological assemblies,” says Zewail.