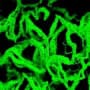
When implanted medical devices like urinary stents and catheters get clogged with biofilms, the usual solution is to take them out and replace them with new ones. Now, however, researchers at the University of Bern and ETH Zurich, Switzerland have developed an alternative. By incorporating ultrasound-activated moving structures into their prototype “stent-on-a-chip” device, they showed it is possible to remove biofilms without removing the device itself. If translated into clinical practice, the technology could increase the safe lifespan of implants, saving money and avoiding operations that are uncomfortable and sometimes hazardous for patients.
Biofilms are communities of bacterial cells that adhere to natural surfaces in the body as well as artificial structures such as catheters, stents and other implants. Because they are encapsulated by a protective, self-produced extracellular matrix made from polymeric substances, they are mechanically robust and resistant to standard antibacterial measures. If not removed, they can cause infections, obstructions and other complications.
Intense, steady flows push away impurities
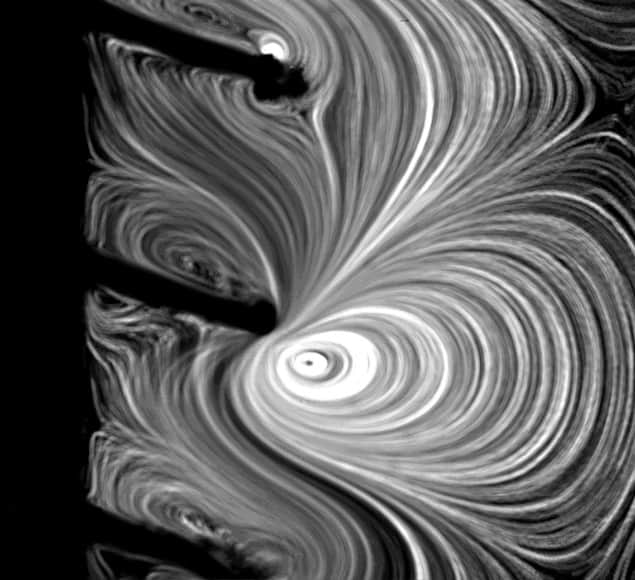
The new technology, which was co-developed by Cornel Dillinger, Pedro Amado and other members of Francesco Clavica and Daniel Ahmed’s research teams, takes advantage of recent advances in the fields of robotics and microfluidics. Its main feature is a coating made from microscopic hair-like structures known as cilia. Under the influence of an acoustic field, which is applied externally via a piezoelectric transducer, these cilia begin to move. This movement produces intense, steady fluid flows with velocities of up to 10 mm/s – enough to break apart encrusted deposits (made from calcium carbonate, for example) and flush away biofilms from the inner and outer surfaces of implanted urological devices.
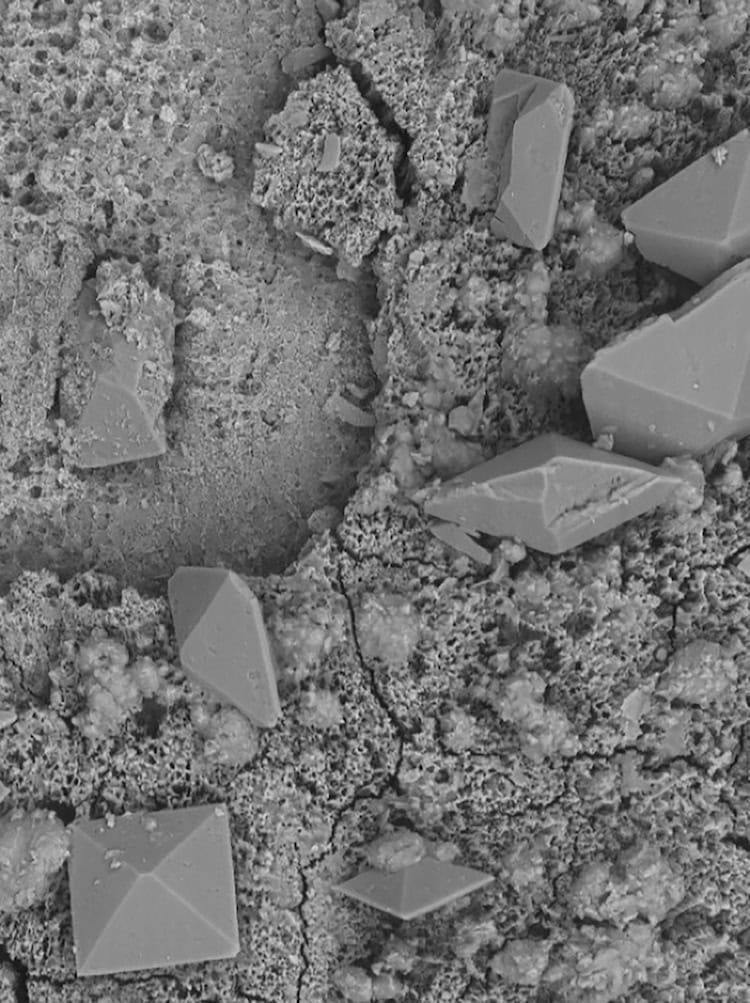
“This is a major advance compared to existing stents and catheters, which require regular replacements to avoid obstruction and infections,” Clavica says.
The technology is also an improvement on previous efforts to clear implants by mechanical means, Ahmed adds. “Our polymeric cilia in fact amplify the effects of ultrasound by allowing for an effect known as acoustic streaming at frequencies of 20 to 100 kHz,” he explains. “This frequency is lower than that possible with previous microresonator devices developed to work in a similar way that had to operate in the MHz-frequency range.”
The lower frequency achieves the desired therapeutic effects while prioritizing patient safety and minimizing the risk of tissue damage, he adds.
Wider applications
In creating their technology, the researchers were inspired by biological cilia, which are a natural feature of physiological systems such as the reproductive and respiratory tracts and the central nervous system. Future versions, they say, could apply the ultrasound probe directly to a patient’s skin, much as handheld probes of ultrasound scanners are currently used for imaging. “This technology has potential applications beyond urology, including fields like visceral surgery and veterinary medicine, where keeping implanted medical devices clean is also essential,” Clavica says.
The researchers now plan to test new coatings that would reduce contact reactions (such as inflammation) in the body. They will also explore ways of improving the device’s responsiveness to ultrasound – for example by depositing thin metal layers. “These modifications could not only improve acoustic streaming performance but could also provide additional antibacterial benefits,” Clavica tells Physics World.
Bacterial ‘cables’ form a living gel in mucus
In the longer term, the team hope to translate their technology into clinical applications. Initial tests that used a custom-built ultrasonic probe coupled to artificial tissue have already demonstrated promising results in generating cilia-induced acoustic streaming, Clavica notes. “In vivo animal studies will then be critical to validate safety and efficacy prior to clinical adoption,” he says.
The present study is detailed in PNAS.