Optogenetics – the use of light to manipulate neurons in the brain – could help scientists better understand the mechanisms underlying brain function, and how neural activity and brain disorders are linked. To fully exploit the potential of this technique, however, requires a way to optically stimulate specific regions throughout the entire brain.
With this aim, a research team headed up in Japan has developed a flexible microLED array film that can adhere to the brain surface and illuminate individual or multiple brain regions for optogenetic stimulation. The researchers describe the device in Applied Physics Express.
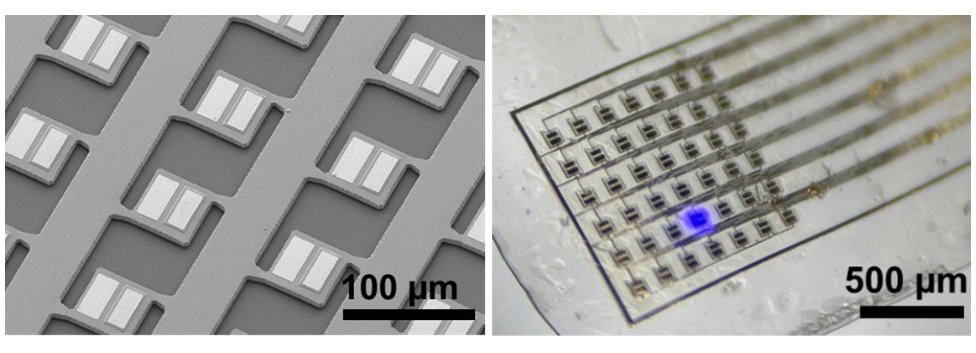
To create the bioimplantable stimulation device, first author Hiroto Sekiguchi from Toyohashi University of Technology developed a way to precisely pattern a layer of microLEDs on an ultrathin flexible film. Sekiguchi and colleagues selected the biocompatible polymer parylene C as the base film, due to its robustness and flexibility. Their first task was to determine the optimal film thickness to balance bendability against fragility. Wrapping experiments investigating the effect of the radius of curvature on film adhesion revealed that a parylene thickness of 11 µm provided sufficient adhesion and robustness.
Next, the researchers established a method for fabricating a high-density, hollow-structured array of indium gallium nitride (InGaN)-based microLEDs. They arranged the 80 x 80 µm microLEDs, which emit 450 nm light, in a 7 × 6 array on a 1 mm square. The hollow structure separates the LED layer from its substrate, enabling the use of a thermal release sheet to transfer a layer of microLEDs onto a parylene film, without rotation or misalignment.

The film-mounted microLEDs generated bright blue emission, which was maintained even when the film was bent. Comparisons of the current–voltage characteristics before and after the transfer process showed that the process had not affected the microLED performance.
Optogenetic investigations require light output values in the range 0.1–1 mW/mm2 to activate channelrhodopsin-2 (ChR2, the first widely adopted optogenetics tool). The researchers note that driving a single microLED at 0.5 mA generated blue light with an output of 22 mW/mm2.
To determine the safety of the optical stimulation with regard to tissue heating, the researchers calculated the temperature increase for a light output of roughly 10 mW/mm2. They observed an increase of 1.6 °C in air – less than the maximum value specified in guidelines for embedded devices (2 °C), suggesting that the device would be safe to use in vivo.
Finally, to test the functionality of the flexible microLED array film, the team placed a film on the surface of the cerebral cortex in an anaesthetized mouse. The 11 μm thick film adhered well to the surface of the brain. Simultaneously driving three microLEDs generated three bright blue emission spots.
Examining the extent of light penetration into the animal’s brain, using electroluminescence measurements and Monte Carlo simulations, revealed that the brain region 100–250 μm away from the surface could be stimulated with 10 mW/cm2 of blue light. This indicates that the flexible microLED film should be effective for optogenetic stimulation.
Wireless implant uses optogenetics to control spinal cord activity in mice
Sekiguchi tells Physics World that the team now plans to develop technology for integrating microLEDs and neural electrodes, which can measure brain waves via electroencephalography. “Also, we think that the next challenge is to integrate microLEDs with different emission colours,” he adds.
“The development of flexible microLED films can significantly contribute to the field of neuroscience research, particularly optogenetic research for the purpose of functional dissection of sub-regions on the brain surface,” the researchers conclude.




