Electronic waste could be transformed from an environmental headache into a literal goldmine thanks to a technique known as flash Joule heating. The technique, which scientists at Rice University in the US have now expanded to include a broader range of materials, can be used to recover valuable metals from waste quickly and simply, without toxic solvents and with much less energy than current laboratory methods. The processed waste also contains a very low concentration of heavy metals, making it safe for agricultural use.
The world’s consumers produce more than 40 million tonnes of electronic waste each year. Since only about 20% of this e-waste is recycled, it is becoming an increasingly serious problem. Most of the rest ends up in landfills, which is disastrous for the environment – not least as it often contains heavy metals such as chromium (Cr), arsenic (As), cadmium (Cd), mercury (Hg) and lead (Pb), some of which are highly toxic.
Sustainable resource
With the right type of processing, however, e-waste could also be a substantial – and sustainable – source of precious metals like rhodium (Rh), palladium (Pd), silver (Ag) and gold (Au). Indeed, the concentrations of some of these elements are actually higher in e-waste than they are in natural ores. For this reason, recovering metals from e-waste – a process known as urban mining – is becoming more cost-competitive with traditional mining.
The drawback is that e-waste recycling processes are far from perfect. The main ones are based on pyrometallurgy, which involves creating a molten soup of metals at high temperatures, and thus lacks selectivity as well as requiring a lot of energy. These methods also produce hazardous, heavy-metal-bearing fumes, especially when the waste contains metals like Hg, Cd and Pb that have relatively low melting points. Other methods rely on hydrometallurgy, in which metals are leached out of e-waste using acids, bases or cyanide. While these methods are more selective, they produce large amounts of (often highly polluted) liquid waste and sludge, and involve kinetically slow chemical reactions, making them hard to scale up. A third family of techniques, known as biometallurgy, involves harnessing biological processes in microorganisms to separate metals, but this promising research is still in its infancy.
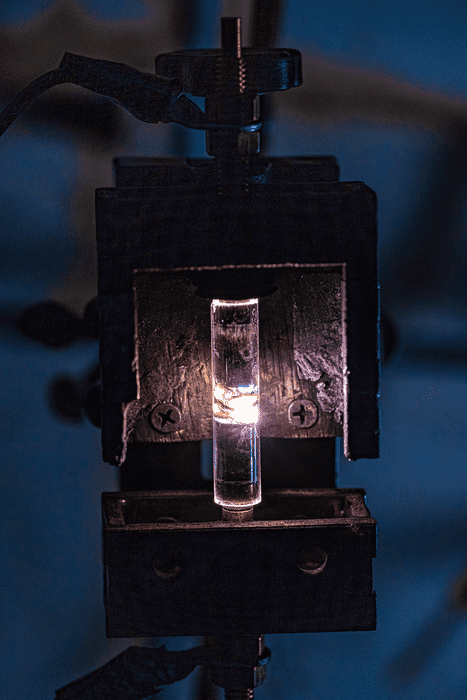
Flash Joule heating
In 2020, a Rice University team led by James Tour developed a way of producing graphene from carbon sources like waste food and plastic. The same team has now adapted this flash Joule heating method to recover metals like Rh, Pd, Au and Ag from e-waste. A further advantage is that the same approach can also remove toxic metals like Cr, As, Cd, Hg and Pb from the waste after the more valuable metals have been extracted.
The technique relies on the fact that the metals in e-waste have vapour pressures that are very different from those of typical substrates such as carbon, ceramics and glass. In a process known as evaporative separation, the researchers vaporize these metals in a flash chamber by applying a brief (less than 1 second), intense pulse of current to the waste, rapidly heating it to 3400 K. The vapours are transported under vacuum from the flash chamber to a cold trap where they condense into their constituent metals, explains team member Bing Deng. The metal mixture in the trap can then be further purified using well-established refining methods.

‘Keyhole surgery’ could reduce environmental burden of metal extraction
The researchers claim that their technique, which they describe in Nature Communications, can recover more than 80% of metals like Rh, Pd and Ag when halide additives are included in the mix, while yields for Au exceeded 60%. They also report that a single flash Joule reaction reduced the concentration of Pb in the remaining char to below 0.05 ppm – the level deemed safe for agricultural soils. Increasing the number of flashes reduced the levels of As, Hg and Cr further, too. “Since each flash takes less than a second, this is easy to do,” Tour says.
The process, which the researchers say is scalable, consumes roughly 939 kilowatt-hours per tonne of e-waste processed. The Rice team say that this is 80 times less energy than commercial smelting furnaces and 500 times less than lab tube furnaces.



