Liver cancer is the second leading cause of cancer death, and radiotherapy is an important treatment option for patients with unresectable tumours. Liver tumours, however, are typically surrounded by healthy liver and dose to the target is limited by the tolerance of this tissue. Proton therapy could provide more conformal dose delivery, thus minimizing high-dose irradiation of normal liver tissue.
“Proton therapy offers advantages over photon-based radiation treatments because it allows sparing of more normal liver volume at low to moderate doses,” explains Harald Paganetti from Massachusetts General Hospital (MGH) and Harvard Medical School. “Radiation therapy for hepatocellular carcinoma is currently being investigated in several clinical trials, including proton therapy.”
Currently, proton therapy is delivered assuming a fixed relative biological effectiveness (RBE) of 1.1. But RBE is dependent on factors including dose, linear energy transfer (LET) and tissue-specific parameters such as α/β. To investigate the impact of different RBE values, Paganetti and colleagues investigated how variable RBE affects normal tissue complication probability (NTCP) and tumour control probability (TCP) in proton therapy of the liver (Phys. Med. Biol. 63 195001).
RBE comparisons
The study included data from 16 liver cancer cases treated with proton therapy at MGH. Twelve patients received either 40 Gy in five fractions or 58.05 Gy in 15 fractions. Four were treated with slightly different schedules that, for comparison, were converted to match the majority.
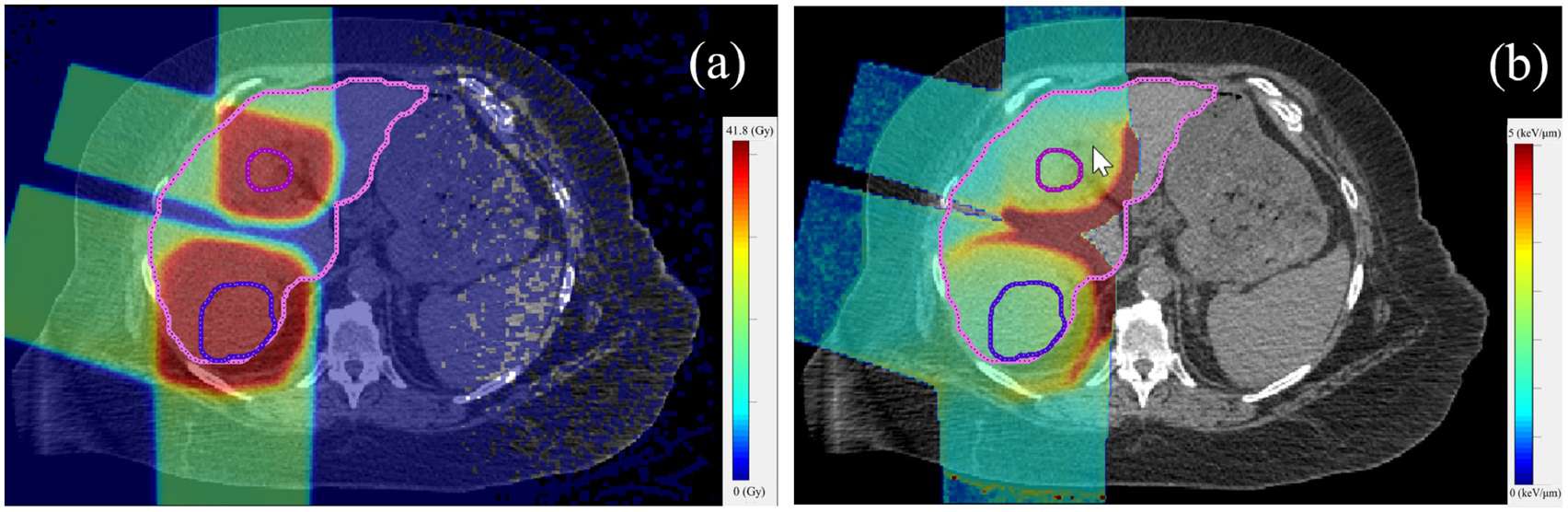
The researchers used the TOPAS Monte Carlo system to calculate dose and LET distributions in the patients. They then utilized an in-house developed empirical model to generate RBE values — calculated as a function of proton physical dose, dose-averaged LET and α/β for photons — and RBE-weighted dose distributions. Finally, they modelled the NTCP of normal liver and TCP of the liver tumour.
The mean RBE-weighted dose and fraction-size equivalent dose (FED, normalized to 1.5 Gy/fraction) differed widely among the 16 patients, due to variation in patient anatomy and radiation fields. This led to considerable differences in NTCP for both RBE scenarios and both fractionation regimens. In all cases, however, NTCP calculated using variable RBE was higher than that using an RBE of 1.1. The size of this NTCP increase depended upon the mean FED in the normal liver.
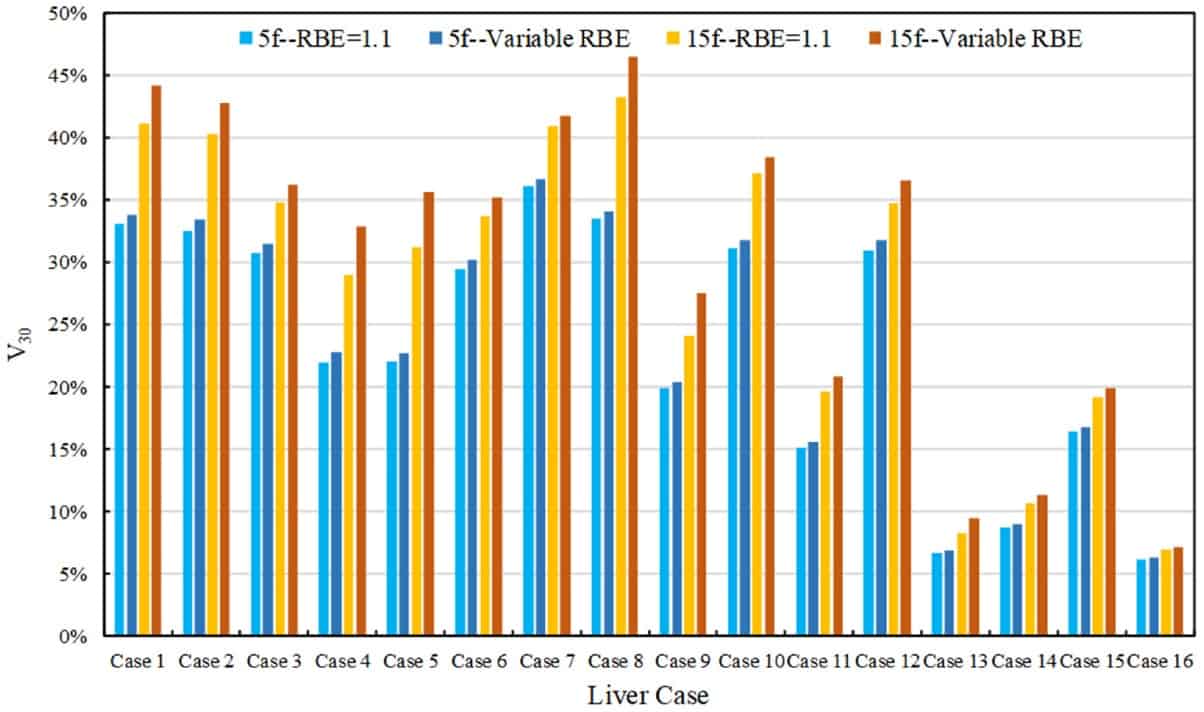
For patients with a mean FED larger than 29.8 Gy, applying the variable RBE model resulted in an NTCP increase averaging 11.6%. For cases with NTCP approaching zero, on the other hand, the impact of variable RBE was negligible. The researchers also examined the volume of normal liver receiving over 30 Gy RBE-weighted dose (V30), a parameter used to predict radiation-induced liver disease. They found that V30 using variable RBE was larger (by 0.1-4.4%) than V30 using RBE of 1.1, for all cases.
The mean RBE-weighted dose to the tumour was about 2.5% less with variable RBE, resulting in a 3-4% smaller TCP in all cases and implying that an RBE of 1.1 may slightly overestimate TCP for liver tumours.
The team also assessed how variable RBE influenced differences in NTCP and TCP between the two fractionation schemes. They found that the impact of variable RBE on differences in NTCP between the two schemes was highly case-specific. For example, variable RBE decreased the NTCP difference from 23.2% to 19.1% for case 1, and increased it from 10.4% to 13.3% for case 12. The difference in TCP between the two fractionation schedules did not change significantly when using variable RBE.
α/β uncertainties
The models employed to calculate variable proton RBE, NTCP and TCP depend on α/β, a tissue-specific parameter that varies among the patient population. As such, the researchers also examined the impact of α/β uncertainty on mean RBE-weighted dose, NTCP and TCP, using α/β values of 2, 2.5 and 3 Gy for normal liver and 7.2, 10 and 15 Gy for liver tumour. For both fractionation schemes, the RBE-weighted dose in normal liver and the target decreased with increasing α/β, as did the NTCP and TCP.
The authors conclude that assuming a constant RBE of 1.1 may overestimate the therapeutic ratio for proton therapy of the liver, mainly due to significant underestimation of NTCP. Paganetti notes that the use of variable RBE would not complicate treatment planning. “If one assigns a homogeneous RBE across the tumour volume, [this is] equivalent to simply adjusting prescription doses,” he explains.
For normal tissues, however, the variation in RBE would typically be larger due to more inhomogeneous doses and LET distributions. “RBE-based treatment planning for normal tissue is currently associated with significant uncertainties and thus not practical,” Paganetti tells Physics World.