Electric fields have been used to produce vortex rings in pure water in an experiment in Sweden. The electric field breaks up the water molecules and the protons released in this process cause the rings to form. The techniques developed in the experiment could be used to mimic the conditions under which chemical processes occur in living cells (Appl. Phys. Lett. 87 153109).

Zackary Chiragwandi and colleagues at Göteburg University and Chalmers University applied an electric field between two gold electrodes covered with pure water and found that the water molecules break up at 3.2 volts. This reaction takes place at both the anode and cathode at the same time. At the anode, the water molecules break up into negative hydroxide (OH–) ions and protons. These protons are released into the bulk of the water, where they create the vortex rings. Meanwhile, the hydroxide ions decompose to form oxygen.
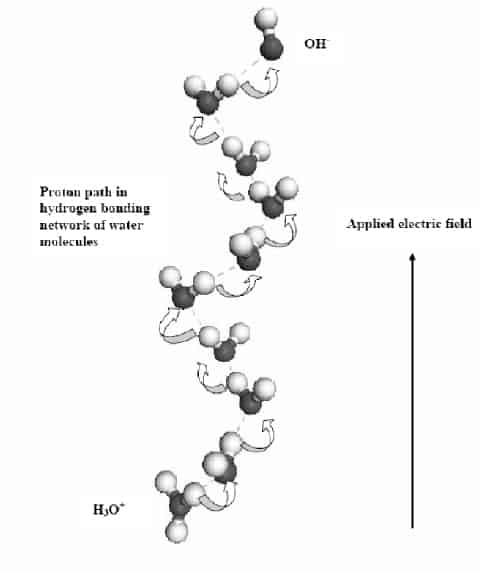
Using an optical microscope, the Swedish team observed that the vortex rings consist of water swirling around in very fine circles with diameters ranging from 10 to 50 microns (figure 1). Moreover, more than one vortex can form at higher voltages. The scientists say that the protons move along a spiral path in solution, which corresponds to the hydrogen-bonding network between water molecules, and that this leads the formation of the vortices (figure 2).
Chiragwandi and co-workers believe that the physics behind the phenomenon is analogous to the transport of electrons in hole-doped semiconductors. The fact that the vortices extend quite deeply into the bulk of the water suggests that they are formed because of a defect at the surface of the anode. The Swedish team has seen such a defect with an electron microscope and also in simulations.
The work could also help improve our understanding of the chemical processes that occur inside living cells and the vortices themselves could be used as a non-invasive way to “stir” aqueous solutions.