Physicists in Germany say they have measured the correlated behaviour of atoms in molecules prepared in their lowest quantum energy state for the first time. Using a technique known as Coulomb explosion imaging, they showed that the atoms do not simply vibrate individually. Instead, they move in a coupled fashion that displays fixed patterns.
According to classical physics, molecules with no thermal energy – for example, those held at absolute zero – should not move. However, according to quantum theory, the atoms making up these molecules are never completely “frozen”, so they should exhibit some motion even at this chilly temperature. This motion comes from the atoms’ zero-point energy, which is the minimum energy allowed by quantum mechanics for atoms in their ground state at absolute zero. It is therefore known as zero-point motion.
Reconstructing the molecule’s original structure
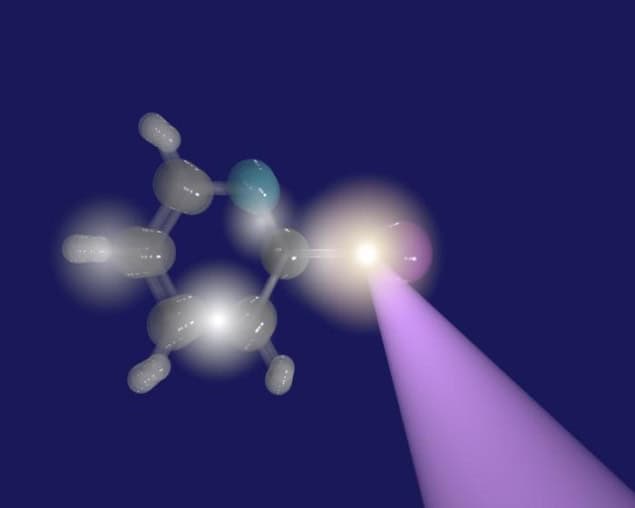
To study this motion, a team led by Till Jahnke from the Institute for Nuclear Physics at Goethe University Frankfurt and the Max Planck Institute for Nuclear Physics in Heidelberg used the European XFEL in Hamburg to bombard their sample – an iodopyridine molecule consisting of 11 atoms – with ultrashort, high-intensity X-ray pulses. These high-intensity pulses violently eject electrons out of the iodopyridine, causing its constituent atoms to become positively charged (and thus to repel each other) so rapidly that the molecule essentially explodes.
To image the molecular fragments generated by the explosion, the researchers used a customized version of a COLTRIMS reaction microscope. This approach allowed them to reconstruct the molecule’s original structure.
From this reconstruction, the researchers were able to show that the atoms do not simply vibrate individually, but that they do so in correlated, coordinated patterns. “This is known, of course, from quantum chemistry, but it had so far not been measured in a molecule consisting of so many atoms,” Jahnke explains.
Data challenges
One of the biggest challenges Jahnke and colleagues faced was interpreting what the microscope data was telling them. “The dataset we obtained is super-rich in information and we had already recorded it in 2019 when we began our project,” he says. “It took us more than two years to understand that we were seeing something as subtle (and fundamental) as ground-state fluctuations.”
Since the technique provides detailed information that is hidden to other imaging approaches, such as crystallography, the researchers are now using it to perform further time-resolved studies – for example, of photochemical reactions. Indeed, they performed and published the first measurements of this type at the beginning of 2025, while the current study (which is published in Science) was undergoing peer review.
Quantum fluctuations are controlled for the first time, say optics researchers
“We have pushed the boundaries of the current state-of-the-art of this measurement approach,” Jahnke tells Physics World, “and it is nice to have seen a fundamental process directly at work.”
For theoretical condensed matter physicist Asaad Sakhel at Balqa Applied University, Jordan, who was not involved in this study, the new work is “an outstanding achievement”. “Being able to actually ‘see’ zero-point motion allows us to delve deeper into the mysteries of quantum mechanics in our quest to a further understanding of its foundations,” he says.