Difficult to diagnose early and frequently deadly when discovered late, oesophageal cancer poses a threat to patients and public health systems alike. Joe McEntee reports on a Europe-wide effort to develop and commercialize better methods of detecting this increasingly common disease

Oesophageal cancer is a bad news story in every sense. The disease occurs in the gullet – the long, hollow tube that helps to move food from the back of the throat to the stomach before digestion – and is currently associated with an extremely poor survival rate: just 10% of patients will still be alive five years after diagnosis in instances where oesophageal cancer is detected late. The number of cases is also on the rise. According to the World Cancer Research Fund International, there were more than 570,000 new cases of oesophageal cancer diagnosed in 2018 – a six-fold increase over three decades – making it both the seventh most common cancer and the sixth biggest cause of cancer deaths worldwide. To make matters worse, oesophageal cancer is one of the most expensive cancers to manage and treat in the oncology clinic, with average costs (over five years) of around €130,000 per patient.
This gloomy picture owes much to the fact that oesophageal cancer is notoriously difficult to detect in its early stages. At this point the disease is eminently treatable: the five-year survival rate jumps to 90% when cancers are caught early. The trouble is that conventional, white-light endoscopy – a visual inspection of the oesophageal tract using a long thin tube with a light source and camera on the end – is not very good at spotting or classifying the lesions that signify the disease in its early stages. Patches of suspect tissue can, of course, be sampled and analysed in the lab, but prescribing random biopsies of the gullet isn’t really a viable solution. Among other drawbacks, the process is time-consuming, covers less than 0.1% of the oesophageal surface, and is prone to sampling errors that miss suspect lesions.
Some good news
So far, so unsatisfactory. For the past two years, however, a cross-disciplinary team of scientists, engineers and clinicians from six European countries has been working to change the narrative for the better. The four-year, €4m ESOTRAC project – funded as part of the European Union’s Horizon 2020 research and innovation programme – is aiming for nothing less than a “paradigm shift” in the early diagnosis of oesophageal cancer. Front and centre to their efforts is a novel hybrid endoscope that combines two biophotonic technologies – optical coherence tomography (OCT) and multispectral optoacoustic tomography (MSOT) – into a single clinical instrument. The overall goal is to make 3D imaging of the oesophageal tract into a routine diagnostic procedure, one that can be carried out in under five minutes and without the need for patient sedation (typically required with current oesophageal endoscopic examinations).
Early detection: variations on the theme
Innovation in biophotonics underpins another promising interrogation scheme for detecting early-stage oesophageal cancer and precancerous lesions. Near-infrared fluorescence molecular endoscopy (NIR-FME) relies on the administration of a fluorescence-labelled antibody (or tracer) that attaches selectively to suspect tissue on the lining of the oesophageal tract. When this tissue is then illuminated at NIR wavelengths, it becomes possible to visualize cancer-specific features both on the surface of the oesophageal tract and just below it. Conventional high-definition white-light endoscopy, in contrast, identifies surface-only morphology and tissue discolourations.
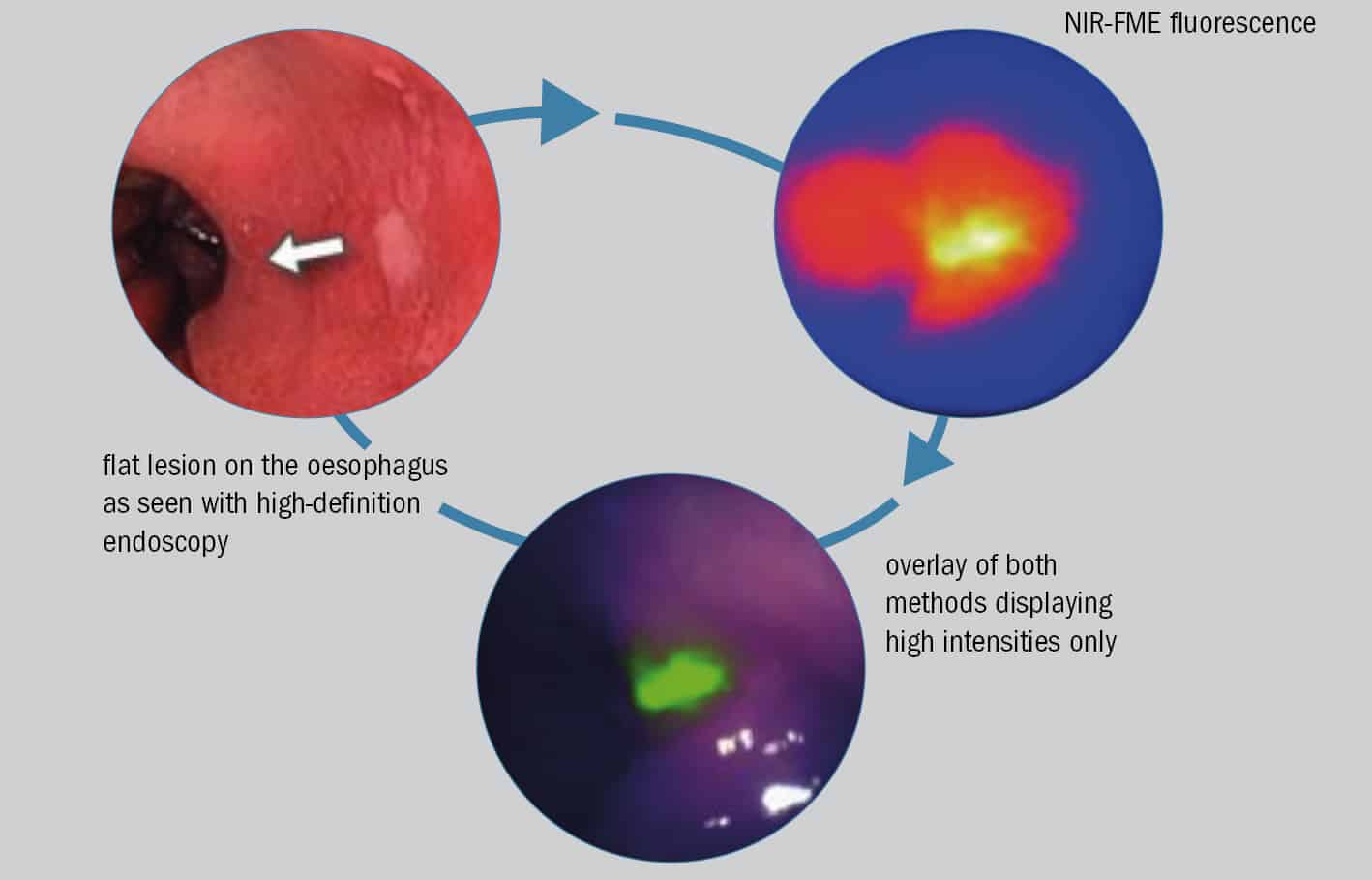
In a paper published in early 2019 (Gut 68 7), a Dutch–German collaboration led by Wouter Nagengast, a clinician at the University of Gröningen and Vasilis Ntziachristos, ESOTRAC project coordinator (see main article), reported on a small-scale clinical trial that highlights the potential of NIR-FME for flagging up oesophageal lesions early. In the study, a cohort of 14 patients, all of them scheduled to undergo a “resection” procedure to remove previously identified cancerous and precancerous lesions from the lining of the digestive tract, were simultaneously evaluated using NIR-FME and high-definition white-light endoscopy.
“Our in vivo results show that even flat and difficult to distinguish lesions were identified with NIR-FME,” the researchers write. What’s more, the subgroup of patients receiving a topical administration of the fluorescent tracer – effectively spraying the tracer onto the oesophageal lining immediately prior to NIR-FME inspection – saw a 33% improvement in early lesion detection compared with conventional white-light endoscopy.
“Fluorescence can guide better evaluation of suspicious lesions,” explains Ntziachristos, adding that the ESOTRAC consortium is investigating whether it might be possible to integrate fluorescence detection into its hybrid endoscope using a forward-looking camera.
If the collaboration hits that mark, the payback for patients (in terms of long-term survival prospects) and cash-strapped public healthcare systems (in terms of aggregate cost savings) will be substantial. “By detecting earlier states of oesophageal cancer, we can shift from high-risk and expensive treatments [surgery and chemotherapy] to simple lesion removal [via resection or ablation],” explains Vasilis Ntziachristos, the project co-ordinator for ESOTRAC and director of the Institute of Biological and Medical Imaging at HelmholtzZentrum München, Germany. “The situation is analogous to early diagnosis of skin cancer,” he adds, “where a simple procedure to remove a melanoma has curable effects, while late-stage disease comes with a very poor prognosis and low five-year survival rates.”
Biophotonic innovation
In January 2019 the ESOTRAC partners (see box above) gathered in Vienna, Austria, for a “half-time” review of their four-year programme. The meeting was essentially a progress report on ESOTRAC’s product development roadmap and, in particular, its stated goal of delivering a “market-ready hybrid endoscope with unparalleled capabilities to detect the early stages of oesophageal cancer”. During the first two years of the project, the partners designed a portable, laboratory-based instrument – essentially a proof-of-principle demonstration to combine MSOT and OCT into a single probe that can acquire complementary tissue information for pathophysiological mapping. In parallel, the teams have also been iterating and optimizing the prototype design for their hybrid endoscope in terms of size, biocompatible materials, optical and ultrasound components, as well as the integration of the core technologies into a practical device.
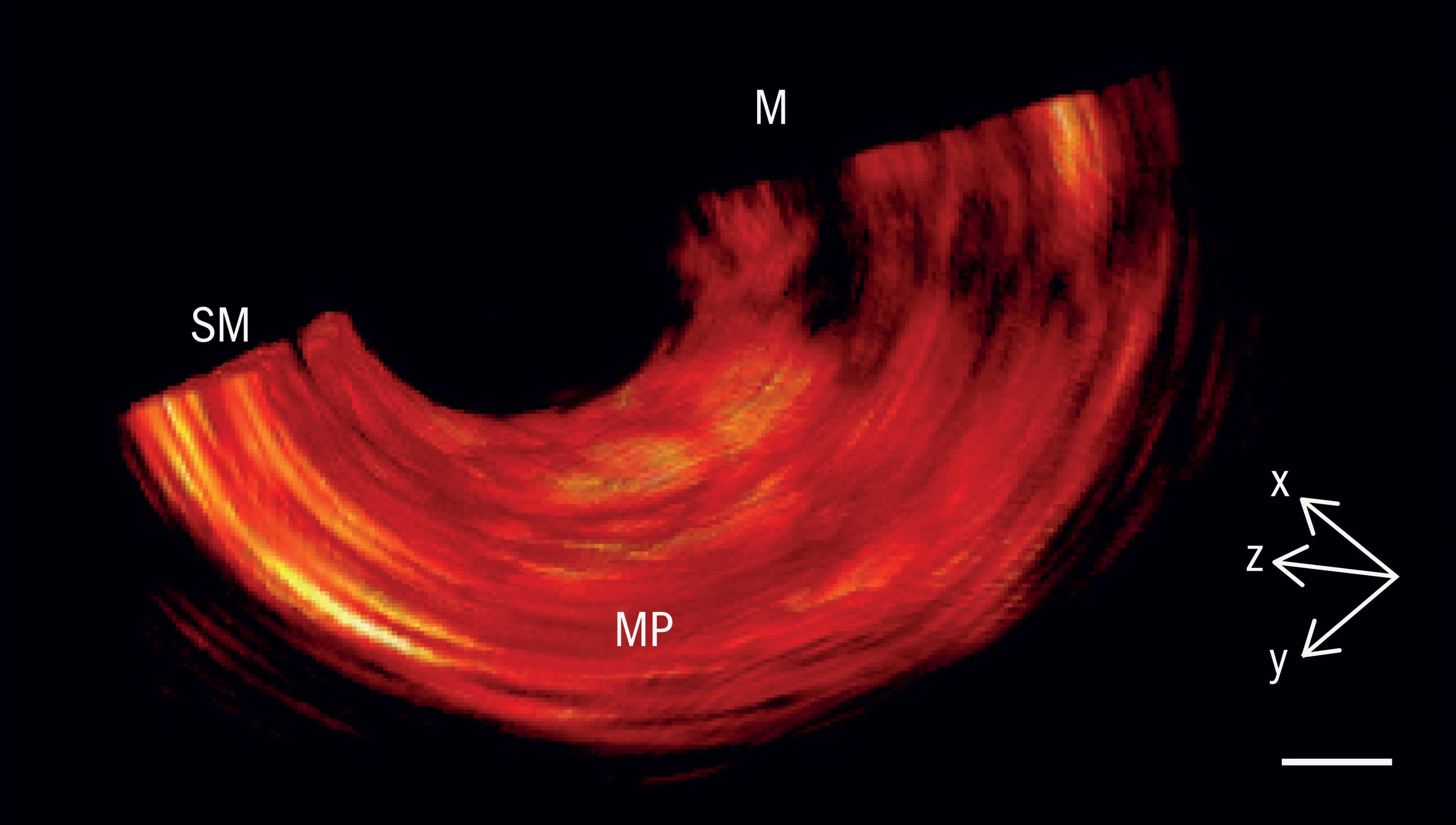
The two imaging modalities chosen for this next-generation endoscope are complementary in nature. MSOT delivers non-ionizing laser pulses into oesophageal tissue. Thanks to the photoacoustic effect, the tissue responds by emitting ultrasonically across a wide frequency band, producing a signal that can be used to identify abnormalities in metabolic activity – for example, changes in blood volume, tissue oxygenation, as well as bleeding and inflammation that are indicative of early-stage cancer. OCT, for its part, uses low-coherence near-infrared interferometry to map reflections from different depths within tissue. Those reflections yield micron-resolution cross-sectional images of subsurface lesions in the oesophagus wall and the growth of new microscopic blood capillaries that feed cancerous tissue.
ESOTRAC in brief
Project budget: €4m
Project duration: 2017–21
Academic partners: Institute of Biological and Medical Imaging, HelmholtzZentrum München, Germany (co-ordinator); University of Cambridge, UK; Technical University of Denmark; Medical University of Vienna, Austria
Industry partners: Sonaxis (France); Amplitude Systèmes (France); Statice (France); Ascenion (Germany); RayFos (UK)
Expertise: optoacoustics, OCT, oesophageal cancer (clinical research), ultrasound probes (manufacturing), ultrafast lasers (manufacturing), software development, biomaterials and biomedical devices (manufacturing), exploitation and intellectual property
As ESOTRAC moves into its third year, the partners’ immediate goal is to optimize the prototype of their hybrid endoscope and to analyse imaging data obtained during studies on “tissue phantoms” (specially designed test objects) and excised oesophageal tissue. Further iteration of the endoscope design will be informed by that image analysis. Ntziachristos calls the endoscope’s development “a photonics-intense and hardware-intense process,” and adds that the team is now finalizing the functional prototype and integrating and miniaturizing the various subcomponents that need to go into it, including optics, ultrasound and motorized control for volumetric scanning. The result, he says, will be “a world-first in terms of integration of optoacoustic and optical coherence tomography in a single endoscope”.
Translational science
From a biomedical perspective, the ESO-TRAC partners know that success will ultimately be measured by whether they can translate their R&D endeavours into a commercial product – ideally one that can be deployed for routine clinical application at scale. With this in mind, the consortium is working to secure patents for many of the project’s core innovations – spanning laser sources, ultrasound detectors, imaging software and the overall hybrid endoscope design. They also expect that, in the long run, the cost of the ESOTRAC-developed technology will be comparable to current commercial endoscopes, which are amortized after around 1300 examinations.
Even more compelling is the bigger picture associated with early detection of oesophageal cancer. A mere 1% increase in the number of people whose cancers are detected early, rather than late, would translate into vastly improved five-year survival rates for close to 6000 patients worldwide. It would also save a heap of money: the €130,000 five-year average cost of treating a patient whose oesophageal cancer was diagnosed late drops to around €12,000 when the disease is caught early, with the potential to yield annual savings in excess of €650m for national healthcare systems.
For the ESOTRAC partners, the end-game couldn’t be clearer. “With the increasing prevalence of oesophageal cancer worldwide, earlier detection has the potential to save tens of thousands of lives every year,” concludes Ntziachristos. “The consortium has big plans for the future, with a prototype that can be robustly deployed in clinical trials and one day translated into clinics everywhere.”
- Enjoy the rest of the 2019 Physics World Focus on Optics & Photonics in our digital magazine or via the Physics World app for any iOS or Android smartphone or tablet.



