Contrary to common belief, the greenhouse effect may have more to do with water in our atmosphere than gases such as carbon dioxide
Extreme variations in local weather and the seasons make it easy for people to mutter “greenhouse effect”, and blame everything on carbon dioxide. Along with other man-made gases, such as methane, carbon dioxide has received a bad press for many years and is uniformly cited as the major cause of the greenhouse effect. This is simply not correct. While increases in carbon dioxide may be the source of an enhanced greenhouse effect, and therefore global warming, the role of the most vital molecule in our atmosphere – water – is rarely discussed. Indeed, water barely rates a mention in the hundreds of pages of the 2001 report by the Intergovernmental Panel on Climate Change.
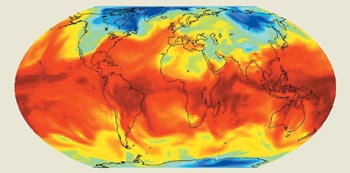
Many aspects of the seemingly simple water molecule conspire to make it difficult to model its effect on our climate. Unlike most other atmospheric gases, the distribution of water in the atmosphere varies strongly with time, location and altitude (figure 1). Water is also unique among atmospheric molecules because it changes phase at terrestrial temperatures. This means that it can transfer energy from its frozen form at the poles to its liquid and vapour forms in the atmosphere. Once in the atmosphere, water moves with the winds and can even diffuse up to the stratosphere, where it is responsible for destroying the ultraviolet-shielding ozone layer.
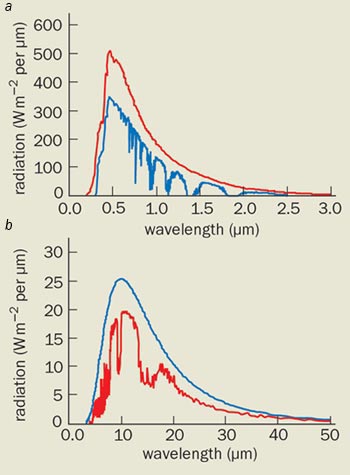
The atmosphere plays a crucial role in the Earth’s radiation budget because it absorbs both the incoming radiation from the Sun and the outgoing radiation that is reflected from the planet’s surface. However, the radiation in each of these processes has very different wavelengths. The Sun radiates approximately as a black body with a temperature of 5800 K, which peaks in the optical region at a wavelength of about 0.6 µm. The reflected radiation profile, on the other hand, is much closer to a black body at a temperature of 275 K, and has a peak at much longer infrared wavelengths (about 11 µm). The physical processes that lead to the absorption of radiation in the two regions are different, but water vapour plays the dominant role in both.
Balancing the books
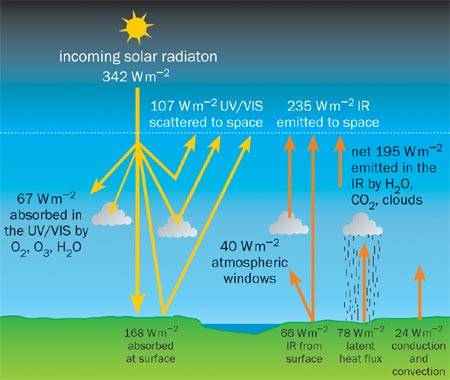
Physicists have been modelling the Earth’s atmosphere for over a century, and we have built up a very detailed understanding of the key processes that are involved in the global energy budget (figure 2). For example, it is now well established that the top of the Earth’s atmosphere receives a surface-averaged energy input from the Sun of 342 W m-2. This is calculated by knowing the amount of energy that is radiated by the Sun and the angle that the Earth subtends. If the incoming and outgoing radiation is not equal then the global energy budget does not balance and the temperature of the planet will change until a new balance is established. What is feared is that a build-up of greenhouse gases is causing an increase in the absorption of the outgoing, infrared radiation.
Satellite measurements show that 235 W m-2 of incoming solar radiation is absorbed by the Earth, but the latest models and measurements suggest that the atmosphere is responsible for just 67 W m-2 of this amount. The rest is absorbed by the ground and by the oceans, which play a key role in the energy budget due to their large heat capacity and their ability to store carbon dioxide, and, of course, water vapour.
The greenhouse effect is precisely the difference between the long-wave radiation that is emitted by the Earth’s surface and the upward thermal radiation that leaves the tropopause – the upper boundary of the turbulent portion of the atmosphere that we all inhabit. The greenhouse effect is about 146 W m-2 in clear skies and some 30 W m-2 higher under cloud cover.
There are a number of popular misconceptions about the greenhouse effect, notably that it is a bad thing. On the contrary, the greenhouse effect is a significant factor in making the Earth habitable. Without it the average temperature on Earth would be lowered by about 30 K, which would make most of the planet’s surface decidedly chilly. Furthermore, it is the water vapour in the lower 10 km or so of the atmosphere, rather than man-made carbon-dioxide emissions, that contributes most to this warming effect.
The absorption of light by molecules in the atmosphere generally results in two basic molecular processes: bound-free and bound-bound transitions. Bound-free transitions take place in the more energetic ultraviolet region of the spectrum and cause the molecules to break up. In bound-bound transitions, which occur at longer wavelengths, the molecules jump from some combination of rotational and vibrational states to another, which produces a very distinct “signature” (figure 3). It is therefore very easy to identify which atmospheric absorbers are at work, although it is much more difficult to work out the actual numbers. Nevertheless, large databases that list all the known molecular transitions and their associated properties have been compiled. The most widely used is the high-resolution transmission molecular absorption database (HITRAN), which has been developed over many years by Larry Rothman, who is now at the Harvard-Smithsonian Center for Astrophysics in Cambridge in the US.
But when the absorption values in the HITRAN database are used in model-atmosphere calculations, the results are disturbing. For clear skies, the models predict that the atmosphere absorbs much less sunlight than is measured by a variety of satellite and aircraft. The difference between the predictions and the measurements can be as large as 30 W m-2. (see “Radiation budget is called to account” by A Maurellis Physics World November 2001 pp22-23). This problem has become known as the absorption anomaly. And there are even worse problems in understanding absorption models when the sky is cloudy.
Not all models underestimate the amount of atmospheric absorption because some physicists choose to add extra absorption to their models to mop up the surplus radiation. However, the physical cause of the missing clear-sky absorption and its exact wavelength distribution remain unresolved, and a source of fertile speculation. Everyone’s favourite molecule is always a candidate.
Our favourite molecule is water. Water vapour is responsible for 70% of the known absorption of incoming sunlight, particularly in the infrared region. Indeed, ask any infrared astronomer about which regions of the spectrum provide the best views and you will get a list of the wavelengths where water does not absorb – the so-called atmospheric windows. After all, there have to be some pretty strong reasons to brave the inhospitable climate of Antarctica to build the South Pole Telescope, as US astronomers have recently undertaken. Water absorption bands are also present in the optical region and extend all the way to the ultraviolet, although they are less strong at shorter wavelengths. The precise effect of these absorption bands is hard to determine, despite the best efforts of many talented and dedicated scientists.