Once upon a time carbon was just another element to physicists, of interest mostly for its ability to perform calculations on the backs of envelopes and other pieces of paper. Even though life itself was based on the sixth element of the periodic table and carbon had many industrial applications, the interests of most physicists lay elsewhere: hydrogen; the various isotopes of helium; semiconductors such as silicon, germanium and gallium arsenide; uranium and so forth. And when the naturally occurring elements were not enough, new ones were created.
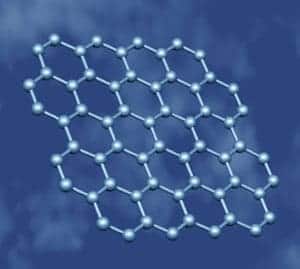
How things have changed. Two breakthroughs in the fairly recent past – the discovery of the fullerenes (starting with carbon-60) in the mid-1980s, followed by the production of the first carbon nanotubes in the early 1990s – have led to an explosion in the number of research papers on the mechanical, electronic and other properties of carbon-based materials.
On page 33 Michael Coey and Stefano Sanvito describe the mysterious magnetic properties of carbon. At first sight carbon seems to be a most unpromising raw material for making magnets. The basic ingredients of any magnet are atoms that contain unpaired electrons, and the six electrons in a typical carbon like to form pairs.
However, ever since a team at the Ioffe Institute in St Petersburg found signs of ferromagnetism in carbon-60 samples in 2001, the evidence that carbon can be magnetic has grown stronger. Indeed, Coey and co-workers have even found evidence for magnetic carbon in fragments from a meteorite that crashed into the Earth 50,000 years ago. Defects are thought to be the most likely source of the magnetism, although this has yet to be confirmed.
This year alone has seen a long list of carbon-based breakthroughs in physics: the use of carbon nanotubes as filaments in light bulbs; a carbon-based foam that has the lowest density ever reported for a solid; nanotube-based sensors that are capable of measuring tiny forces; ballistic electron transport in layers of graphite just an atom thick; and the use of single carbon atoms to improve the resolution of atomic force microscopes to better than 0.1 nm (see physicsweb.org/articles/world/17/11/1 for links to these and other stories).
Moreover, the results are not restricted to condensed-matter and device physics. Japanese physicists have used carbon-60 molecules to change the radioactive half-life of beryllium nuclei, while carbon-70 molecules are being employed in experiments in Austria to explore the boundary between quantum and classical mechanics.
Of course, some physicists were interested in carbon long before fullerenes and nanotubes arrived on the scene. In the 1950s, for instance, Fred Hoyle famously predicted the existence of a previously unknown excited state of the carbon nucleus to explain how elements could be formed inside stars. The state was found in experiments shortly afterwards. The search for carbon-based life forms in space has been less successful, but the current enthusiasm for all things carbon on Earth shows little sign of fading away.