Scientists have succeeded in imaging a single-electron wavefunction or "orbital" in a molecule for the first time. David Villeneuve of the National Research Council of Canada (NRC) in Ottawa and colleagues used femtosecond lasers to reconstruct the highest occupied molecular orbital in nitrogen molecules (J Itatani et al. 2004 Nature 432 867). These electrons are responsible for the chemical properties of molecules.
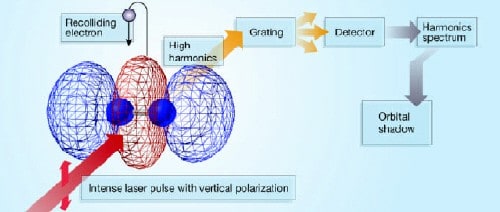
“Most techniques for studying molecular structure, such as X-ray diffraction and electron scattering measure the total electron distribution,” Villeneuve told PhysicsWeb. “We were able to isolate a single orbital from among the many in the nitrogen molecule. Furthermore, we detected the wavefunction itself rather than the more usual square of the wavefunction.”
Villeneuve and colleagues from the NRC, the University of Ottawa, the INRS lab in Varennes and the Japan Science and Technology Agency used two laser pulses in their experiments: the first aligned the molecules in a chosen direction, while the second — which had a duration of 30 femtoseconds (30 x 10-15 seconds) — removed an electron from the highest occupied molecular orbital. About 1.3 femtoseconds later the electric field of the second laser changed direction, which caused the electron to accelerate back towards the parent molecule and collide with it. This released an energetic X-ray photon that could be detected.
By changing the angle between the molecule and the laser beam and repeating the experiment, the scientists were able to build up a 3D image of the molecular orbital. This involved developing a mathematical model to relate the X-ray emission spectrum to the shape of the molecular orbital. The model was similar to those used in medical tomography.
“Since the images are recorded in only about 30 femtoseconds, we can also resolve simple molecular processes such as dissociation,” said Villeneuve. “We now hope to be able to see the character of the orbital change as the molecule breaks apart. This may shed more light on how molecular bonds are made and broken in chemical reactions.” It might also be possible to observe the motion of electrons on attosecond (10-18 seconds) time-scales with the technique.