New experiments in Japan have confirmed that liquids can exist in more than two different states at the same time -- contrary to what is commonly believed. Yoshinori Katayama and colleagues at the Synchrotron Research Center in Hyogo found that phosphorus contains both a low-pressure fluid phase and a high-pressure polymer liquid phase, while Rei Kurita and Hajime Tanaka of the University of Tokyo saw evidence for two different liquid states in triphenyl phosphite.
A liquid can co-exist with its solid or gas form: water, for instance, can exist alongside ice in some situations and steam in others. However, recent research has suggested that different liquid phases might also be able to co-exist. These phases have the same chemical composition, but different atomic or molecular structures, and are known as “polymorphs”.
Katayama and colleagues found that molten phosphorus shows an abrupt structural change between two stable disordered states at a temperature of about 1000 degrees centigrade and a pressure of around 1 gigapascal. In situ X-ray diffraction experiments at the SPring-8 synchrotron radiation source showed that the change is reversible and that the two structures coexist during the transformation (Science 306 848). The team believes that the material switches between a dense molecular form containing tetrahedral phosphorus molecules (P4) at low pressures, and a polymer form with a much lower density at higher pressures.
“Such a transition is extremely rare in pure materials and has only ever been confirmed in helium-3 at low temperatures, but this is due to a quantum effect,” Katayama told PhysicsWeb. “A first-order transition between low-density and high-density amorphous ice is also well known, but the amorphous state is not thermodynamically stable.”
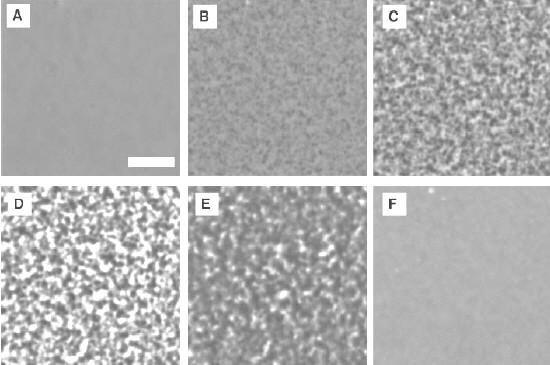
Meanwhile, Kurita and Tanaka used phase-contrast optical microscopy to show that the simple molecular fluid triphenyl phosphite can transform into a “glacial” phase at temperatures above 480 degrees centigrade. Although the transformation between the two liquids took place quite slowly (see figure), the Tokyo team believes that there is a critical point at which it occurs (Science 306 845). This point could depend on the build up of long-range correlations between the molecules.
“Our findings suggest that a liquid-liquid transition is not just specific to some unusual liquids, but may, in principle, exist in any liquid,” said Tanaka.