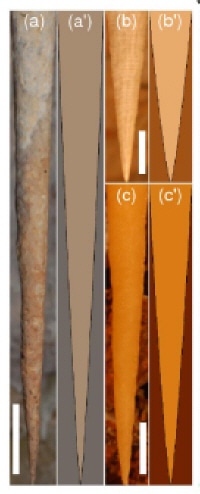
Physicists often go underground to shield their experiments from cosmic rays but a group of researchers in the US has recently been underground for another reason -- to study the growth of stalactites in caves. Raymond Goldstein and colleagues of the University of Arizona have developed a model which predicts that all stalactites basically have the same shape. Observations at the nearby Kartchner Caverns State Park confirmed that real stalactites do indeed have this shape (M B Short et al. 2005 Phys. Rev. Lett. 94 018501).

Stalactites form when water containing carbon dioxide and limestone (calcium carbonate) drips into an underground cave through cracks in the ground. Since the partial pressure of carbon dioxide inside the cave is lower than that in the rock, the carbon dioxide escapes from the water droplets. As a result, the limestone comes out of solution and is deposited on the ceiling of the cave. This process ultimately creates a stalactite that continues to grow over a period of hundreds of years.
In the model developed by Goldstein and co-workers the rate at which calcium carbonate leaves the solution is proportional to the thickness of the water film flowing down the surface of the stalactite. The rate at which the surface grows is also a function of the local radius and the slope of the stalactite surface.
To test their model Goldstein and co-workers “grew” artificial stalactites on a computer, and found that the final shape of the stalactite was independent of the starting shape. Moreover, the shape created by the model is the same as that observed in real stalactites (figure 2).
“Our work lays the foundation for understanding the formation of a whole range of structures found in limestone caves,” said Goldstein. “These structures, in turn, have historical records of climate variation locked in them. It also advances our general understanding of the physics and chemistry of precipitation, which underlies many other natural formations, such as hydrothermal vents.”
The Arizona group is now working on more exotic shapes found in caves, including “draperies” and “crenulations”.