Researchers in the US have made a new device capable of detecting cancer cells and viruses. The device could eventually be developed into low-cost tests for doctors to use in developing countries where expensive diagnostic equipment is hard to come by, says team leader Mehmet Toner at Massachusetts General Hospital.
It is usually a challenge to detect single cancer cells in a blood sample because they are present in very small amounts – just a few cells per millilitre of sample. These “circulating” cancer cells, as they are known, are those that have broken away from the original tumour site and indicate that a cancer has metastasized, decreasing a patient’s chance of survival. It is thus crucial to be able to detect them. “Of all deaths from cancer, 90% are not the result of cancer at the primary site but from tumours that have spread from the original site,” explained team member Brian Wardle at Massachusetts Institute of Technology (MIT).
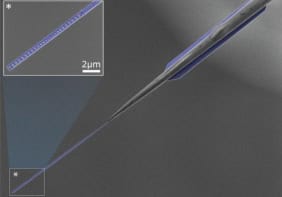
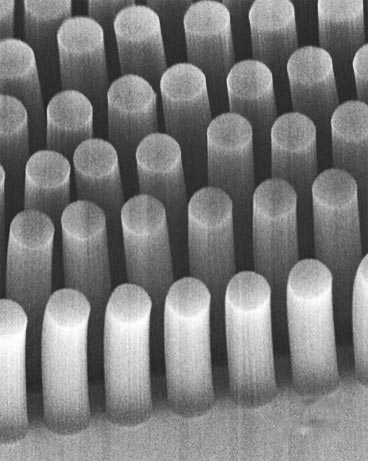
Toner and colleagues had made an earlier version of their device four years ago that consisted of thousands of nanometre-sized silicon “posts” confined inside microfluidic channels. The posts were coated (or “functionalized”) with antibodies that preferentially stick to certain types of tumour cell. When a blood sample was passed through the device (at around 2 ml per hour), tumour cells in the sample that came into contact with the posts became trapped.
Porosity is the key
However, the problem with this configuration was that some cancer cells passed through the device without contacting the posts at all. Toner’s team has now overcome this problem by making the posts porous instead of solid. In this way, the cells flow through the posts as well as around them. This means the cells have a greater chance of touching the posts and sticking to them.
The new porous posts were designed by Wardle, who is an aeronautics engineer. Such nano-engineered structures are usually studied with the goal of making advanced composite material for stronger aircraft parts.
The posts in the new device are made of vertically aligned carbon nanotubes instead of silicon and can collect cancer cells eight times better than the solid posts, say the researchers. Carbon nanotubes are rolled up sheets of carbon atoms and assemblies of the tubes are highly porous. Indeed, an array of carbon nanotubes – which can contain up to 100 billion carbon nanotubes per square centimetre – is 99% air.
As in the original device, the researchers functionalize the surface of each nanotube with different antibodies that stick to a particular type of cancer cell. But that’s not all: changing the configuration of the nanotubes also allows them to capture different sized objects – for example, cells that are around 10 µm in diameter, all the way down to viruses, which can be as small as just 40 nm.
Coated in antibodies
Toner and Wardle’s team has proved that its device works by detecting a variety of bacteria and viruses, including fluorescent labelled Streptococcus pneumoniae bacteria. The researchers did this by coating the micofluidic device with anti-S. Pneumoniae antibody. They also succeeded in detecting human leukocytes, taken from healthy volunteers, and coating the devices with anti-CD4 antibodies.
“Sample preparation and filtering is crucial when working directly with complex biological samples, such as blood,” commented Hatice Altug of Boston University, who was not involved in the work. “In this respect, it is very exciting to see that this device can very effectively capture and separate target bioparticles across multiple size scales, ranging from viruses to bacteria and cells.”
And, since the technology is also compatible with large-area and low-cost manufacturing, it could find a wide range of applications in diagnostics, she added.
The MIT team is now working on tailoring the device so that it can detect HIV, the virus that causes AIDS.
The current work was detailed in Small DOI:10.1002/smll.201002076.