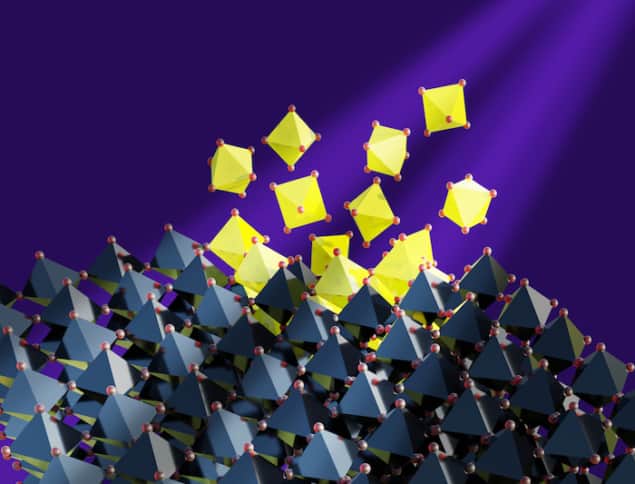
For the first time, researchers in the UK and Japan have identified the locations where materials known as perovskites begin to degrade. This degradation, which is caused by the formation of defects that act as “traps” for charge carriers moving through the material’s crystalline structure, is a barrier to commercializing perovskite-based devices such as solar cells because it reduces their efficiency over time. The new work could therefore help improve the stability and performance of perovskites by pointing the way toward controlling the defects’ formation.
Metal halide perovskites have an ABX3 structure, where A is typically caesium, methylammonium (MA) or formamidinium (FA); B is lead or tin; and X is chlorine, bromine or iodine. They are good candidates for thin-film solar cells because they can absorb light over a broad range of wavelengths in the solar spectrum. Indeed, the materials now boast a power conversion efficiency (PCE) of more than 25% for single-junction cells and nearly 30% for tandem perovskite/silicon cells. This means they rival established solar-cell materials such as silicon, gallium arsenide and cadmium telluride. They are also potentially cheaper to process than crystalline silicon and can be prepared as a liquid ink that is printed to produce a thin film.
Double trouble
Perovskites’ big drawback is their naturally-occurring defects. These defects are double trouble, because as well as trapping photo-excited charge carriers (electrons and holes), it turns out they are also the sites at which the light-absorbing layer of perovskites begins to undergo photochemical degradation.
In the new work, researchers at the University of Cambridge in the UK and the Okinawa Institute of Science and Technology (OIST) in Japan studied the nanostructure of perovskite thin films and how this changed over time when they were exposed to sunlight. In collaboration with the Diamond Light Source and the electron Physical Sciences Imaging Centre (ePISC) in Didcot, UK, together with the Department of Materials Science and Metallurgy in Cambridge, the team used several high spatial-resolution techniques to show that even trace amounts of defects compromise the material’s stability (and therefore its longevity). The degradation around these defects proceeds much faster than in the surrounding pristine material, and degraded films also contain morphological holes that form around the defects.
Controlling the impurities
The researchers report that the type of defects and the way they are distributed in the materials depends on the composition of the perovskite thin film and how it was processed. Performance losses and intrinsic degradation processes can therefore be mitigated by controlling these impurities, which requires the local structural and chemical properties to be carefully managed.
“We know that structural tuning (tilting of the perovskite lattice) can inhibit the formation of the most detrimental defective phases,” explains Stuart Macpherson, a PhD student at Cambridge and the lead author of a paper in Nature describing the team’s findings. “In fact, this effect has been unknowingly employed in some highly stable devices in the literature.”
Perovskite solar cell survives the damp and heat
Macpherson tells Physics World that eradicating these defective features would bring a twofold benefit in the form of increased efficiency as well as longevity. “Advancing this strategy and identifying passivation agents that have a similar effect will be a natural follow-up for this work,” he adds.
The Cambridge-Okinawa team is now extending its characterization toolkit to continue investigating the impact of defect phases in other perovskite materials. “We are also striving to optimize the device fabrication process based on our findings, to make solar cells that are both efficient and stable,” Macpherson says. “Our future work will focus on probing the fundamental influence of impurity phases on physical processes within perovskite solar cells.”