Expansion microscopy is a biological imaging technique that enables nanoscale imaging using a conventional diffraction-limited fluorescence microscope. It works by embedding samples in a water-swellable hydrogel and then expanding the gel. This physically expands the biomolecules away from each other, enabling their interrogation at a resolution previously only achievable using expensive high-resolution imaging techniques.
Current expansion microscopy protocols, however, are not optimized for widespread adoption. Samples must be treated with custom anchoring agents to link specific biomolecules and labels to the hydrogel. In addition, most approaches have only achieved roughly four-fold tissue expansion, restricting the effective resolution to around 70 nm on a conventional optical microscope with a 280 nm diffraction-limited objective lens.
To overcome these shortcomings, a team headed up at Carnegie Mellon University has developed a novel expansion microscopy strategy called Magnify. The protocol, described in Nature Biotechnology, uses a new mechanically sturdy hydrogel that retains a spectrum of biomolecules without requiring a separate anchoring step.
Magnify can expand specimens by up to 11 times, enabling imaging of cells and tissues with an effective resolution of around 25 nm using a conventional microscope. When combined with super-resolution optical fluctuation imaging (SOFI, a computational post-processing method), it achieved an effective resolution of around 15 nm.
Previous expansion microscopy protocols also required the elimination of many biomolecules that hold tissues together. “To make cells really expandable, you need to use enzymes to digest proteins, so in the end, you had an empty gel with labels that indicate the location of the protein of interest,” explains senior author Yongxin Zhao in a press statement.
“One of the main selling points for Magnify is the universal strategy to keep the tissue’s biomolecules, including proteins, nucleic acids and carbohydrates, within the expanded sample. The molecules are kept intact, and multiple types of biomolecules can be labelled in a single sample,” Zhao adds.
Broad applications
Zhao and colleagues applied Magnify to a wide range of tissue types. Imaging an 11-fold expanded mouse brain section stained for total protein content, for example, enabled visualization of the nanoscopic architecture of individual synapses in the brain. Magnify demonstrated an effective resolving power of around 18 nm using a ×60 objective lens (around 200 nm diffraction limit).
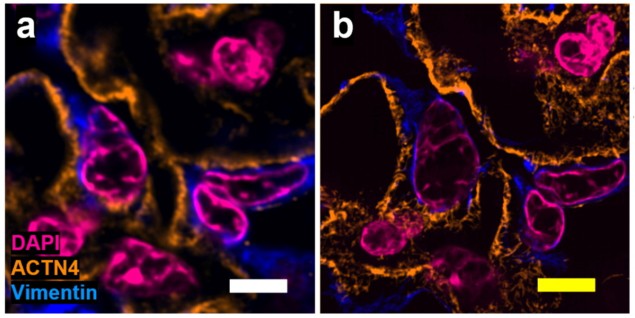
The researchers confirmed the low distortion obtained by the Magnify protocol on several tissue types, using SOFI pre-expansion and confocal microscopy post-expansion. They found no substantial morphological changes between pre- and post-expansion images of cell nuclei and protein markers at either macroscopic or sub-diffraction levels.
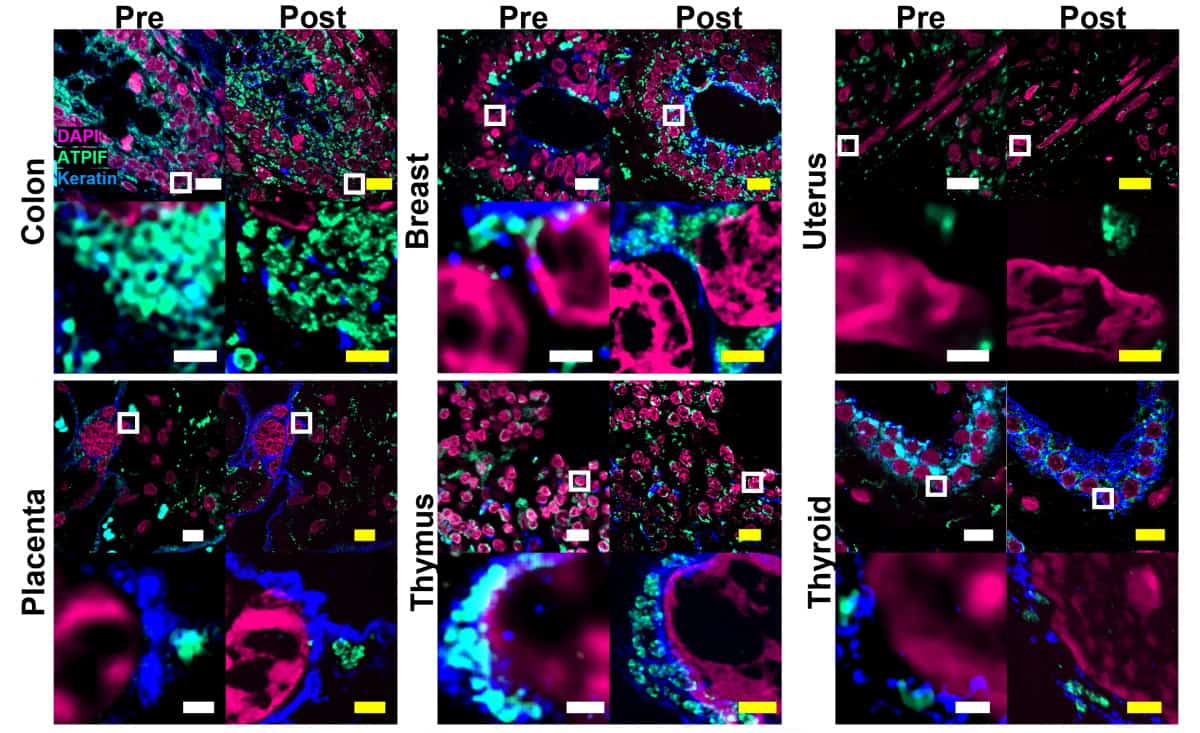
The team also tested Magnify on a range of formalin-fixed paraffin-embedded specimens – which are among the most important biopsy preparations, but are challenging to expand with current protocols. This included tissue sections from kidney, breast, brain and colon, and corresponding tumours. Magnify could expand the samples by factors of around 8.00–10.77 in water, depending on tissue type.
One key goal was to make Magnify suitable for a broad range of tissue specimens, easing its uptake by researchers looking to adopt the new protocol. “It works with different tissue types, fixation methods and even tissue that has been preserved and stored,” says co-first author Brendan Gallagher. “It is very flexible, in that you don’t necessarily need to redesign experiments with Magnify in mind completely; it will work with what you have already.”
Ramping the resolution
To demonstrate the further increase in effective resolution made possible by pairing Magnify with SOFI, the researchers used the combination to image human lung organoids, in particular, the cilia that function to clear mucus in the airway. At 200 nm in diameter and just a few micrometres in length, these structures are usually too small to see without using technology such as electron microscopy (EM).
Magnify–SOFI could fully resolve the hollow structure of cilia and basal bodies, including the outer ring previously shown by EM to comprise nine bundles of microtubules. The researchers estimated the effective resolution as around 14–17 nm (using a 280 nm diffraction-limited objective lens). They were also able to visualize defects in cilia in lung cells with genetic mutations.

Super-resolution microscopy reveals coronavirus-replicating machinery
“With the latest Magnify techniques, we can expand those lung tissues and start to see some ultrastructure of the motile cilia even with a regular microscope, and this will expedite both basic and clinical investigations,” comments co-author Xi Ren.
Building upon the successful development of Magnify, the team is now using it to study even more complex tissue samples. “This includes exploring infected tissues as well as larger specimens such as entire organs,” Zhao tells Physics World. “Moreover, we are working towards optimizing Magnify for investigating pathological human samples and studying nanoscale changes in the brain during learning processes and diseases. With these breakthroughs, further discoveries can be expected from this highly promising field of study.”