In 2023 medical tech firm Occuity won a business start-up award from the Institute of Physics for its ground-breaking ophthalmic and disease-screening technologies. Alistair Bounds explores the power of the eye in screening for diseases and introduces the company’s work developing advanced methods to detect chronic diseases

More than 13 million eye tests are carried out in the UK each year, making it one of the most common medical examinations in the country. But what if eye tests could tell us about more than just the health of the eye? What if these tests could help us spot some of humanity’s greatest healthcare challenges, including diabetes, Alzheimer’s or heart disease?
It’s said that the eye is the “window to the soul”. Just as our eyes tell us lots about the world around us, so they can tell us lots about ourselves. Researchers working in what’s known as “oculomics” are seeking ways to look at the health of the body, via the eye. In particular, they’re exploring the link between certain ocular biomarkers (changes or abnormalities in the eye) with systemic health and disease. Simply put, the aim is to unlock the valuable health data that the eye holds on the body (Chronic Disease. Ophthalmol. Ther. 13 1427).
Oculomics is particularly relevant when it comes to chronic conditions, such as dementia, diabetes and cardiovascular disease. They make up most of the “burden of disease” (a factor that is calculated by looking at the sum of the mortality and morbidity of a population) and account for around 80% of deaths in industrialized nations. We can reduce how many people die or get ill from such diseases through screening programmes. Unfortunately, most diseases don’t get screened for and – even when they do – there’s limited or incomplete uptake.
Cervical-cancer screening, for example, is estimated to have saved the lives of one in 65 of all British-born women since 1950 (Lancet 364 249), but nearly a third of eligible women in the UK do not attend regular cervical screening appointments. This highlights the need for new and improved screening methods that are as non-intimidating, accessible and patient-friendly as a trip to a local high-street optometrist.
Seeing the light: the physics and biology of the eye
In a biological sense, the eye is fantastically complex. It can adapt from reading this article directly in front of you to looking at stars that are light-years away. The human eye is a dynamic living tissue that can operate across six orders of brightness magnitude, from the brightest summer days to the darkest cloudy nights.
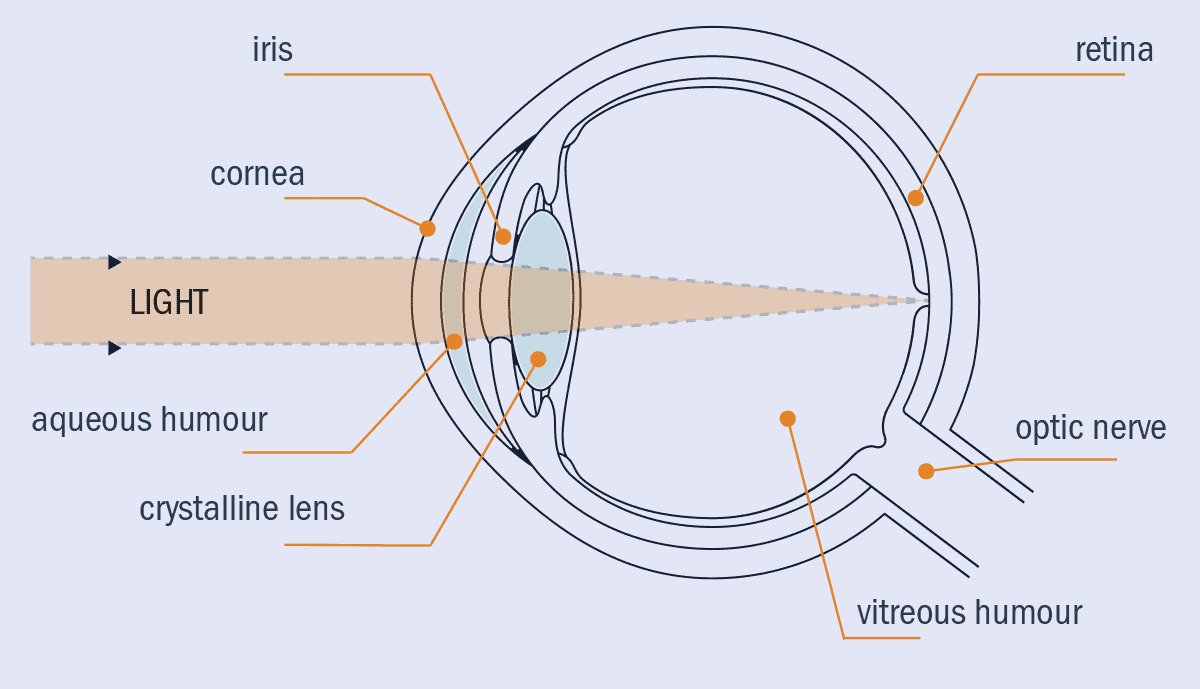
The eye has several key structures that enable this (figure 1). At the front, the cornea is the eye’s strongest optical component, refracting light as it enters the eye to form an image at the back of the eye. The iris allows the eye to adapt to different light levels, as it changes size to control how much light enters the eye. The crystalline lens provides depth-dynamic range, changing size and shape to focus on objects nearby or far away from the eye. The aqueous humour (a water-like fluid in front of the lens) and the vitreous humour (a gel-like liquid between the lens and the retina) give the eye its shape, and provide the crucial separation over which the refraction of light takes place. Finally, light reaches the retina, where the “pixels” of the eye – the photoreceptors – detect the light.
1 Look within
The anatomy of the human eye, highlighting the key structures including the iris, cornea, the lens and the retina.
The tissues and the fluids in the eye have optical characteristics that stem from their biological properties, making optical methods ideally suited to study the eye. It’s vital, for example, that the aqueous humour is transparent – if it were opaque, our vision would be obscured by our own eyes. The aqueous humour also needs to fulfil other biological properties, such as providing nutrition to the cornea and lens.
To do all these things, our bodies produce the aqueous humour as an ultrafiltered blood plasma. This plasma contains water, amino acids, electrolytes and more, but crucially no red blood cells or opaque materials. The molecules in the aqueous humour reflect the molecules in the blood, meaning that measurements on the aqueous humour can reveal insights into blood composition. This link between optical and biological properties is true for every part of the eye, with each structure potentially revealing insights into our health.
Chronic disease insights and AI
Currently, almost all measurements we take of the eye are to discern the eye’s health only. So how can these measurements tell us about chronic diseases that affect other parts of the body? The answer lies in both the incredible properties of the eye, and data from the sheer number of eye examinations that have taken place.
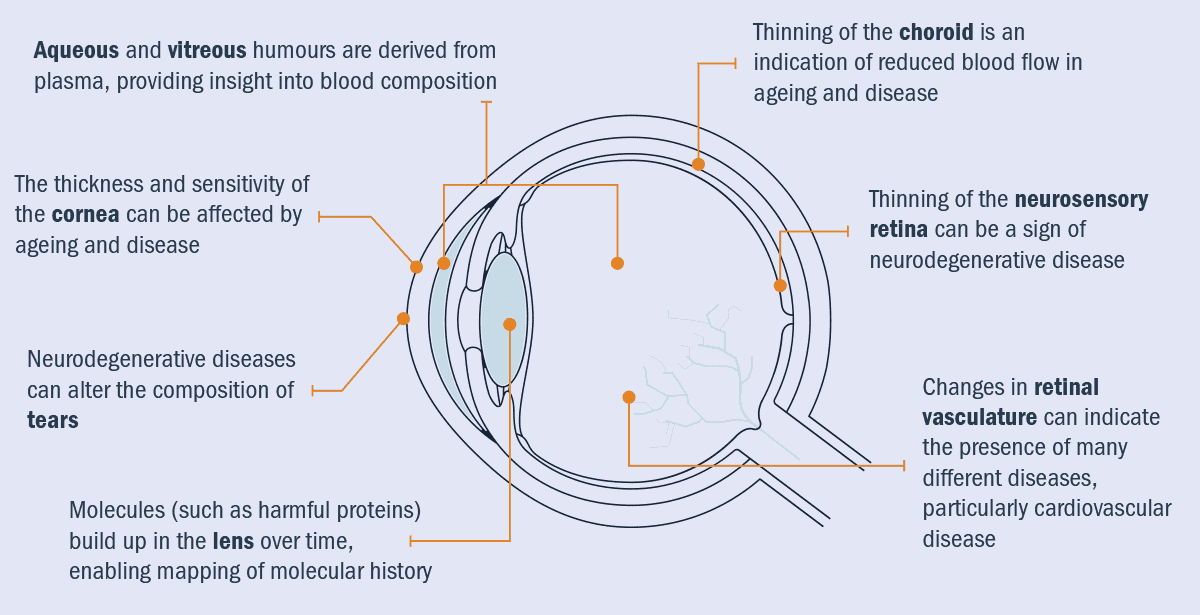
Chronic diseases can affect many different parts of the body, and the eye is no exception (figure 2). For example, cardiovascular disease can change artery and vein sizes. This is also true in the retina and choroid (a thin layer of tissue that lies between the retina and the white of the eye) – in patients with high blood pressure, veins can become dilated, offering optometrists and ophthalmologists insight into this aspect of a patient’s health.
For example, British optometrist and dispensing optician Jason Higginbotham, points out that throughout his career “Many eye examinations have yielded information about the general health of patients – and not just their vision and eye health. For example, in some patients, the way the arteries cross over veins can ‘squash’ or press on the veins, leading to a sign called ‘arterio-venous nipping’. This is a possible indicator of hypertension and hardening of the arteries.”
Higginbotham, who is also the managing editor of Myopia Focus, adds that “Occasionally, one may spot signs of blood-vessel leakage and swelling of the retinal layers, which is indicative of active diabetes. For me, a more subtle sign was finding the optic nerves of one patient appearing very pale, almost white, with them also complaining of a lack of energy, becoming ‘clumsier’ in their words and finding their vision changing, especially when in a hot bath. This turned out to be due to multiple sclerosis.”
2 Interconnected features
Imaging the eye may reveal ocular biomarkers of systemic disease, thanks to key links between the optical and biological properties of the eye. With the emergence of oculomics, it may be possible – through a standard eye test – to detect cardiovascular diseases; cancer; neurodegenerative disease such as Alzheimer’s, dementia and Parkinson’s disease; and even metabolic diseases such as diabetes.
However, precisely because there are so many things that can affect the eye, it can be difficult to attribute changes to a specific disease. If there is something abnormal in the retina, could this be an indicator of cardiovascular disease, or could it be diabetes? Perhaps it is a by-product of smoking – how can an optometrist tell?
This is where the sheer number of measurements becomes important. The NHS has been performing eye tests for more than 60 years, giving rise to databases containing millions of images, complete with patient records about long-term health outcomes. These datasets have been fed into artificial intelligence (AI) deep-learning models to identify signatures of disease, particularly cardiovascular disease (British Journal of Ophthalmology 103 67; J Clin Med. 10.3390/jcm12010152). Models can now predict cardiovascular risk factors with accuracy that is comparable to the current state-of-the-art. Also, new image-analysis methods are under constant development, allowing further signatures of cardiovascular disease, diabetes and even dementia to be spotted in the eye.
But bias is a big issue when it comes to AI-driven oculomics. When algorithms are developed using existing databases, groups or communities with historically worse healthcare provision will be under-represented in these databases. Consequently, the algorithms may perform worse for them, which risks embedding past and present inequalities into future methods. We have to be careful not to let such biases propagate through the healthcare system – for example, by drawing on multiple databases from different countries to reduce sensitivities to country-specific bias.
Although AI oculomics methods have not yet moved beyond clinical research, it is only a matter of time. Ophthalmology companies such as Carl Zeiss Meditec (Ophthalmology Retina 7 1042) and data companies such as Google are developing AI methods to spot diabetic retinopathy and other ophthalmic diseases. Regulators are also engaging more and more with AI, with the FDA having reviewed at least 600 medical devices that incorporate AI or machine learning across medical disciplines, including nine in the ophthalmology space, by October 2023.
Eye on the prize
So how far can oculomics go? What other diseases could be detected by analysing hundreds of thousands of images? And, more importantly, what can be detected with only one image or measurement of the eye?
Ultimately, the answer lies in matching the imaging technique to the disease. It is critical to choose the measurement technique that fits the disease. So, if we want to detect more diseases, we need more measurement techniques.
At Occuity, a UK-based medical technology company, we are developing solutions to some of humanity’s greatest health challenges through optical diagnostic technologies. Our aim is to develop pain-free, non-contact screening and monitoring of chronic health conditions, such as glaucoma, myopia, diabetes and Alzheimer’s disease (Front Aging Neurosci.13 720167). We believe that the best way that we can improve health is by developing instruments that can spot specific signatures of disease. This would allow doctors to start treatments earlier, give researchers a better understanding of the earliest stages of disease, and ultimately, help people live healthier, happier lives.
Currently, we are developing a range of instruments that target different diseases by scanning a beam of light through the different parts of the eye and measuring the light that comes back. Our first instruments measure properties such as the thickness of the cornea (needed for accurate glaucoma diagnosis); and the length of the eyeball, which is key to screening and monitoring the epidemic of myopia, which is expected to affect half of the world’s population by 2050. As we advance these technologies, we open up opportunities for new measurements to advance scientific research and clinical diagnostics.
Looking into the past
The ocular lens provides a remarkable record of our molecular history because, unlike many other ocular tissues, the cells within the lens do not get replaced as people age. This is particularly important for a family of molecules dubbed “advanced glycation end-products”, or AGEs. These molecules are waste products that build up when glucose levels are too high. While present in everybody, they occur in much higher concentrations in people with diabetes and pre-diabetes – people who have higher blood-glucose levels and are at high risk of developing diabetes, but largely without symptoms. Measurements of a person’s lens AGE concentration may therefore indicate their diabetic state.
Fortunately, these AGEs have a very important optical property – they fluoresce. Fluorescence is a process where an atom or molecule absorbs light at one colour and then re-emits light at another colour – it’s why rubies glow under ultraviolet light. The lens is the perfect place to look for these AGEs, as it is very easy to shine light into the lens. Luckily, a lot of this fluorescence makes it back out of the lens, where it can be measured (figure 3).
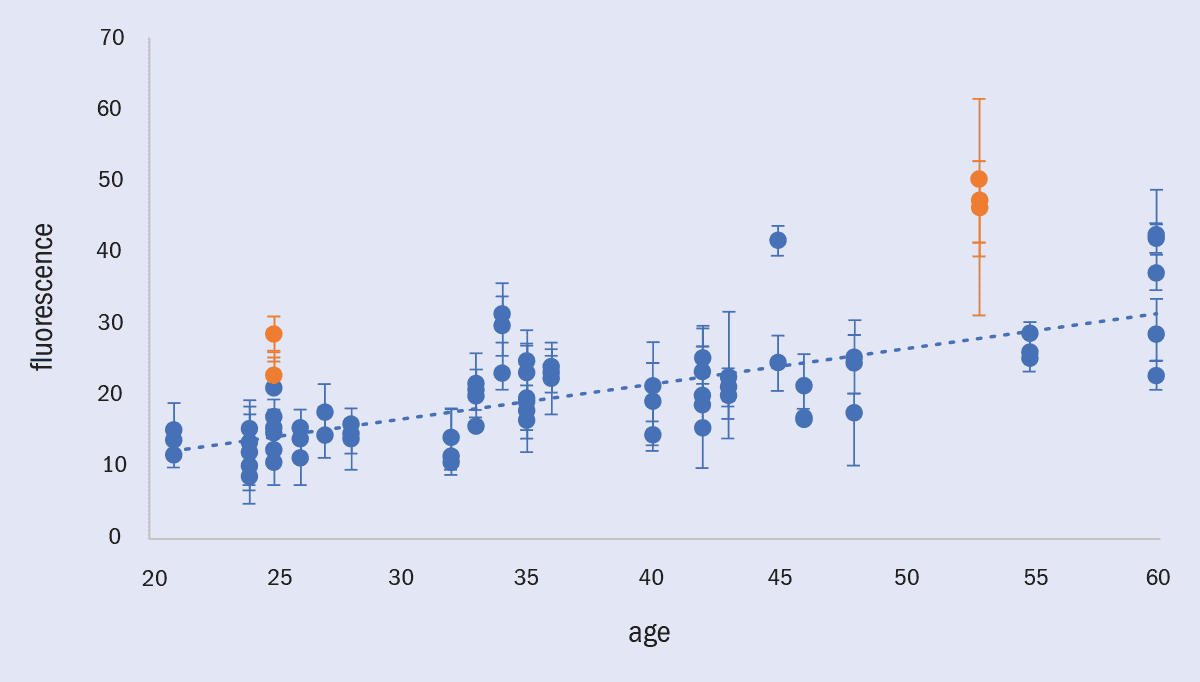
3 AGEs and fluorescence
Fluorescence, a measure of advanced glycation end-products (AGE) concentration, rises as people get older. However, it increases faster in diabetes as higher blood-glucose levels accelerate the formation of AGEs, potentially making lens fluorescence a powerful tool for detecting diabetes and pre-diabetes. This chart shows rising fluorescence as a function of both age and diabetic status, taken as part of an internal Occuity trial on 21 people using a prototype instrument; people with diabetes are shown by orange points and people without diabetes are shown by blue points. Error bars are the standard deviation of three measurements. These measurements are non-invasive, non-contact and take just seconds to perform.
Occuity has developed optical technologies that measure fluorescence from the lens as a potential diabetes and pre-diabetes screening tool, building on our optometry instruments. Although they are still in the early stages of development, the first results taken earlier this year are promising, with fluorescence clearly increasing with age, and strong preliminary evidence that the two people with diabetes in the dataset have higher lens fluorescence than those without diabetes. If these results are replicated in larger studies, this will show that lens-fluorescence measurement techniques are a way of screening for diabetes and pre-diabetes rapidly and non-invasively, in easily accessible locations such as high-street optometrists and pharmacists.
Such a tool would be revolutionary. Almost five million people in the UK have diabetes, including over a million with undiagnosed type 2 diabetes whose condition goes completely unmonitored. There are also over 13 million people with pre-diabetes. If they can be warned before they move from pre-diabetes to diabetes, early-stage intervention could reverse this pre-diabetic state, preventing progression to full diabetes and drastically reducing the massive impact (and cost) of the illness.
Living in the present
Typical diabetes management is invasive and unpleasant, as it requires finger pricks or implants to continuously monitor blood glucose levels. This can result in infections, as well as reduce the effectiveness of diabetes management, leading to further complications. Better, non-invasive glucose-measurement techniques could transform how patients can manage this life-long disease.
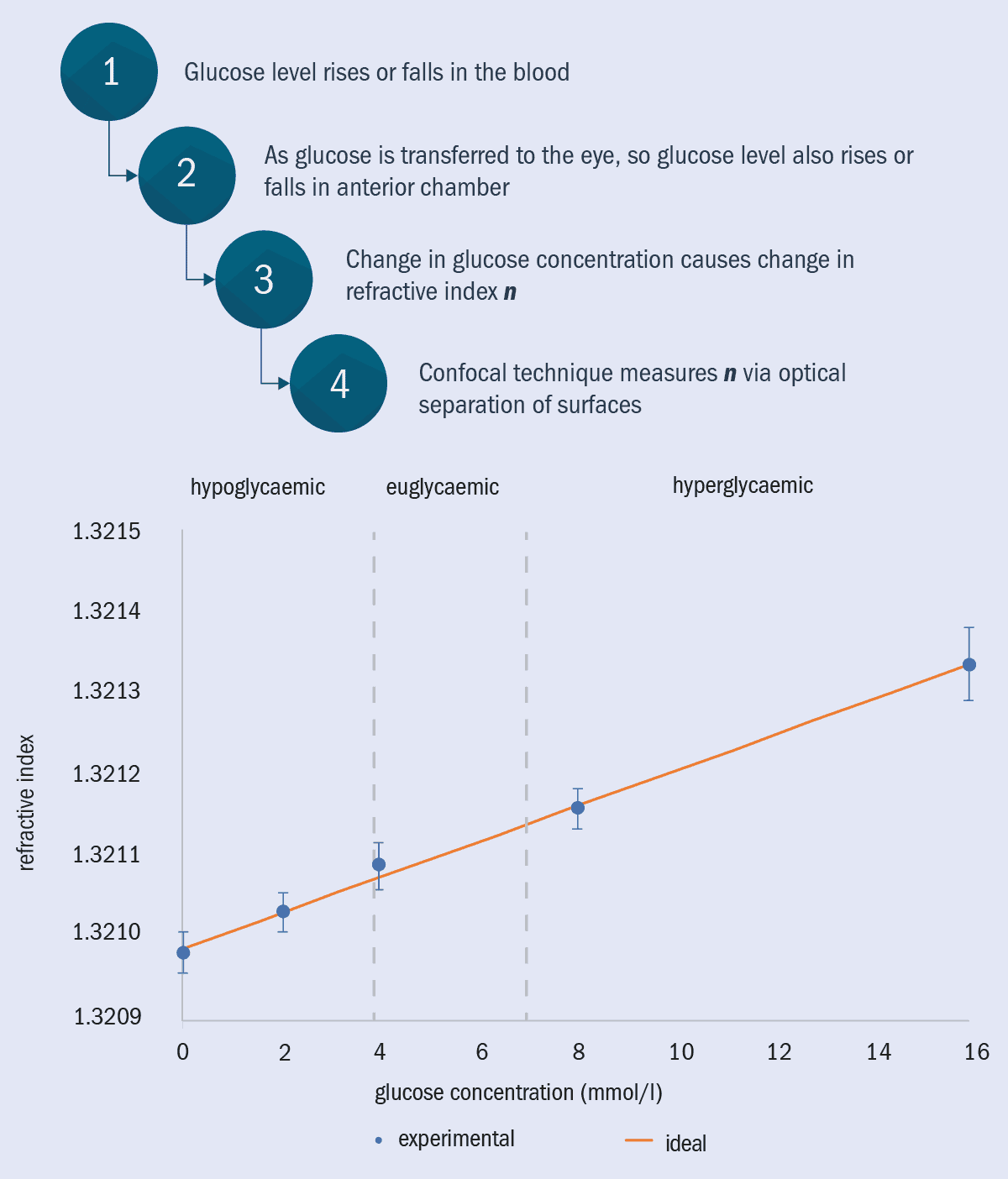
As the aqueous humour is an ultra-filtered blood plasma, its glucose concentration mimics that of the glucose concentration in blood. This glucose also has an effect on the optical properties of the eye, increasing the refractive index that gives the eye its focusing power (figure 4).
4 Measuring blood glucose level
The relationship between blood glucose and optical measurements on the eye has been probed theoretically and experimentally at Occuity. Their goal is to create a non-invasive, non-contact measure of blood glucose concentration for diabetics. Occuity has shown that changes in glucose concentration comparable to that observed in blood has a measurable effect on refractive index in cuvettes and is moving towards equivalent measurements in the anterior chamber.
As it happens, the same techniques that we at Occuity use to measure lens and eyeball thickness can be used to measure the refractive index of the aqueous humour, which correlates with glucose concentration. Preliminary cuvette-based tests are close to being precise enough to measure glucose concentrations to the accuracy needed for diabetes management – non-invasively, without even touching the eye. This technique could transform the management of blood-glucose levels for people with diabetes, replacing the need for repetitive and painful finger pricks and implants with a simple scan of the eye.
Eye on the future
As Occuity’s instruments become widely available, the data that they generate will grow, and with AI-powered real-time data analysis, their predictive power and the range of diseases that can be detected will expand too. By making these data open-source and available to researchers, we can continuously expand the breadth of oculomics.
Oculomics has massive potential to transform disease-screening and diagnosis through a combination of AI and advanced instruments. However, there are still substantial challenges to overcome, including regulatory hurdles, issues with bias in AI, adoption into current healthcare pathways, and the cost of developing new medical instruments.
Despite these hurdles, the rewards of oculomics are too great to pass up. Opportunities such as diabetes screening and management, cardiovascular risk profiling and early detection of dementia offer massive health, social and economic benefits. Additionally, the ease with which ocular screening can take place removes major barriers to the uptake of screening.
With more than 35,000 eye exams being carried out in the UK almost every day, each one offers opportunities to catch and reverse pre-diabetes, to spot cardiovascular risk factors and propose lifestyle changes, or to identify and potentially slow the onset of neurodegenerative conditions. As oculomics grows, the window to health is getting brighter.