Arrays of optical traps that can hold tiny objects with nanometre precision have been created by researchers in the US. Based on a silicon chip, the traps are powered by a single laser and are much smaller, more stable and more energy-efficient than conventional optical traps. The traps can also be set up in less time and do not require a high level of expertise from the operator. As a result, their inventors believe that they could become a useful tool for high-throughput studies of large biological molecules such as DNA.
Optical traps – or optical tweezers – use laser light to manipulate objects that are as tiny and delicate as a single strand of DNA. While these experiments can deliver valuable information about the mechanical properties of large molecules – such as how proteins fold – they can take years to set up and are time consuming and difficult to run.
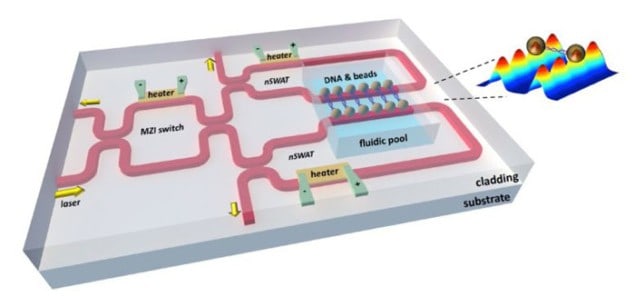
But now a team led by Michelle Wang, and Michal Lipson at Cornell University has created a tiny chip-based device that allows several trapping experiments to run in parallel. Dubbed the nanophotonic standing-wave array trap (nSWAT), the device offers high degrees of stability and control and requires less laser light than conventional schemes.
Plug-in-and-play traps
“Our optical trapping innovation reduces bench-top optics to a small device on a chip,” says Wang, who is a molecular biophysicist. “This is more like plug-in-and-play,” she adds. “For an experiment that typically takes a year to do, we hope to reduce that time to a month.”
Wang and Lipson developed the device along with researchers at Cornell’s physics department and the Howard Hughes Medical Institute. An on-chip interferometer is used to split and recombine an evanescent light wave into two counter-propagating waves that form a standing wave within a waveguide. Evanescent waves occur near the interface of two optical materials and decay rapidly over distances much smaller than the wavelength of the light. This rapid change in intensity is ideal for trapping objects and the antinodes of the standing wave provide an array of traps for tens to hundreds of particles.
The nSWAT uses much less laser power when many traps are run in parallel. “Usually if you want 100 traps you need 100 beams,” explains Wang. “Here the laser beam is recycled – to have more traps you just lengthen the waveguide without needing more laser power.”
Nanometre control
The technique also allows the position of the particles to be controlled with nanometre precision. This is done using a tiny on-chip heater that can change the refractive index of the waveguide. This affects the phase of the evanescent wave, which shifts the position of the antinode traps.
According to Wang, this is unlike previous nanophotonic waveguides in which the trapped particles are continuously propelled along the waveguide with no way of precisely controlling their position. But she adds, “These previous designs served as inspirations for our current work.”
The new approach to trapping is also inherently stable, and video tracking revealed no drift in the position of the trapped particle after more than 10 minutes. Previous attempts to set up multiple optical traps based on time-shared lasers have suffered from poor stability because the laser beam was not steady.
Applications in cell biology
Wang believes that the technique offers a promising approach for single-molecule studies relating to DNA and DNA-based processes, RNA and RNA-based processes, and protein folding and unfolding. She also says there could be applications in cell biology.
“Our challenges now are to optimize every aspect of the devices to make single-molecule measurements routine and streamlined,” says Wang. “This requires substantial work to improve measurement and manipulation capabilities, dovetail the device to commercial microscopes and develop software to fully automate the data acquisition and control.”
Full details are reported in Nature Nanotechnology.
- There is much more about how optical tweezers are used to study biological molecules in this feature article: “Optical tweezers: where physics meets biology”.
- This article first appeared on nanotechweb.org.



