Using a high-speed video camera, physicists in Belgium have watched “antibubbles” form, move and then burst in a liquid for the first time. Stéphane Dorbolo and colleagues at the University of Liège made the antibubbles in a variety of liquids – including soapy water and beer – and their results could lead to a deeper understanding of the physics of fluids (S Dorbolo et al. 2003 New J. Phys. 5 161). Although they were first observed in 1932, little is known about how antibubbles are created or how they collapse.
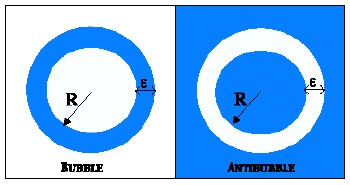
A bubble is a spherical film of liquid that surrounds a pocket of air, and which is in turn surrounded by air itself. An antibubble, as its name suggests, is a spherical shell of air with liquid on both its inside and outside (figure 1).
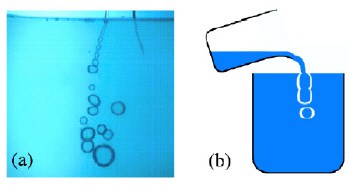
To make their antibubbles, Dorbolo and colleagues slowly poured a small amount of a solution made of soap and water over the surface of a large glass tray that contained the same liquid. They observed that a jet of liquid globules formed beneath the surface, and that this jet then broke up to form a stream of antibubbles that lasted up to two minutes (figure 2).
The antibubbles then collapsed in a way that is similar to the way that ordinary bubbles burst (figure 3). Dorbolo and co-workers say that both the formation and collapse of the antibubbles are due to fluid instabilities – the so-called “Rayleigh-Plateau” and “Rychtmeyer-Meshkov” instabilities.
The team also created antibubbles in salt solutions and in beer “for fun”.