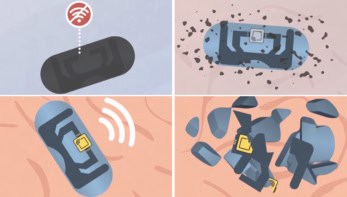
There is currently great interest in creating efficient cell-targeted drug delivery systems that can provide an alternative to existing treatments such as chemotherapy. Promising emerging options include peptide amphiphiles, which are lipid-conjugated peptides that form spherical hydrophobic structures called peptide amphiphile micelles (PAMs). Due to the hydrophilic surface and hydrophobic core, these structures can easily be loaded with a variety of small molecule drugs. In addition, they can be efficiently used to accomplish cell-specific delivery.
Given the desirable modularity of this system, Bret Ulery and his team at the University of Missouri have combined peptide amphiphiles with antitail amphiphiles, lipid-conjugated DNA fragments, to yield novel biomaterials. These combination micelles can be readily fused, or annealed, with an aptamer, a DNA sequence with highly specific molecular targeting, which can be used to preferentially associate with desired cell populations. The resulting micellar platform, termed Aptamer~A/PAM, has been recently shown capable of differentially targeting leukaemia cells (Physical Biology 10.1088/1478-3975/aadb68).

Stable and specific micelles
After fabricating micelles, the researchers demonstrated that they were sufficiently stable in various biological fluids and small enough to be taken up and processed by the cells. Such stability and internalization capacity enables this new device to target a very specific group of cells and then disassemble and release the desired drug to them. This supports their potential utility as a clinical drug delivery system.
The team also provided evidence that micelles specifically interacted with NALM6 cells, a leukaemic cell line. First, the researchers observed that the micelles effectively targeted this cell line. Then, when they introduced a minimal modification in the aptamer, the research team observed that micelle binding to the cells decreased dramatically. Moreover, after testing another similar cell line (LM138) as a negative control, the researchers observed no specific association of their Aptamer~A/PAMs. All of these experiments showed the specificity of their new biomolecular material.
Great potential
The successful synthesis and desirable properties of the engineered technology support the potential of the Aptamer~A/PAM system to serve as an alternative approach for drug delivery. The cell specificity of this system could overcome the drawbacks of leukaemia treatments such as chemotherapy by limiting damage to non-target cells. Moreover, this technology is not limited to this specific cancer, since micelles can be customized for other clinical applications by employing other bioactive peptides and different aptamers.
Although this study shows promising results and represents an important advancement in PAM drug delivery, further work will reveal the full potential of the Aptamer~A/PAM technology. Future studies carried out by Ulery and his team will focus on better understanding the interaction of cells with the platform and its performance in vivo.