Researchers from the Massachusetts Institute of Technology have shown that combining light and sonic pulses can reduce symptoms related to Alzheimer’s disease (AD) and improve cognitive functions. The mechanisms behind this phenomenon are still being explored (Cell 10.1016/j.cell.2019.02.014).
Not much is known about the course of events leading to Alzheimer’s disease, but the formation of toxic β-amyloid plaques and phosphorylated tau proteins have long been described as major hallmarks of the disease. Many treatment approaches are currently being investigated, such as the use of immunogenic therapies, autoimmune responses to the disease, and even machine learning to find potential treatment targets. Li-Huei Tsai and her colleagues have an alternative strategy: trying to hijack the brain to induce specific brain waves.
In a previous study, the team from the Picower Institute for Learning and Memory showed that non-invasive 40 Hz light flicker induced gamma oscillations, a type of brain wave associated with several high-order cognitive functions that are impaired in Alzheimer’s disease. In mice that were genetically predisposed to develop Alzheimer’s disease symptoms, flashing this light for one hour a day reduced their levels of β-amyloid plaques and tau proteins, while boosting the activity of microglia, the immune cells responsible for clearing the brain of residual waste. The researchers named this therapy GENUS: gamma entrainment using sensory stimulus.
Expanding GENUS to auditory signals…
With most of the improvements limited to the visual cortex, the researchers built on these findings to expand GENUS to auditory signals and target new regions of the brain, such as the cortex linked with memory.
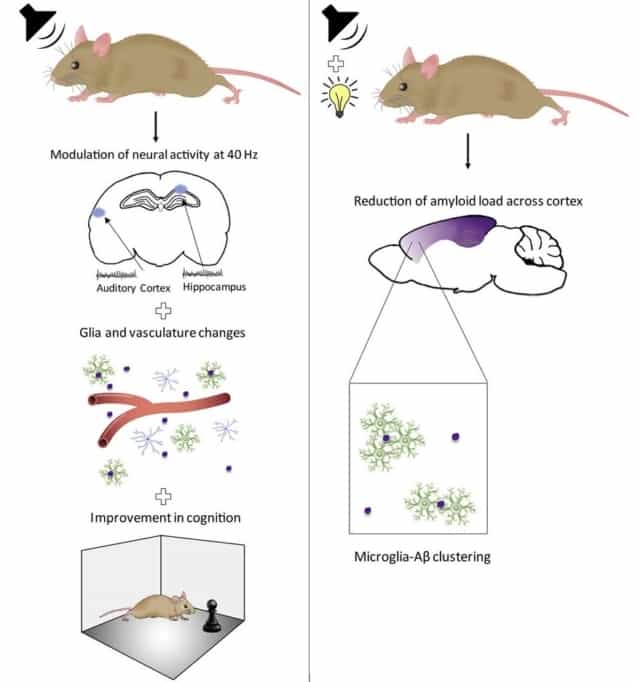
Firstly, they verified that auditory GENUS was efficient enough to fire up neurons in regions-of-interest of the brain. They exposed the mice to trains of tones repeating at various frequencies for one hour a day over seven days and recorded neural activity in different parts of the cortex. This experiment suggested that 40 Hz auditory stimulation does induce GENUS robustly in the main brain parts considered, notably those controlling learning and memory.
The animals’ behavioural and physiological responses corroborated these results. Indeed, when mice had to recognize or locate new objects, or find their way in a water maze, the GENUS group exhibited better performance than a control group of mice exposed to random frequency sound.
Physiologically, GENUS reduced β-amyloid plaques in the auditory cortex and hippocampus, the brain centre for learning and memory. The team suggest that this clearance is enabled by the observed increase in microglia cells and change of vasculature through vasodilatation to flush residues. Finally, GENUS mice also displayed a lower level of hyperphosphorylated tau protein.
… and combining it with visual signals
Since both visual and auditory stimulation induced better cognitive function in treated mice, the final step that researchers took was to combine both types of stimuli and observe how mice responded. The effects of the combined GENUS were even greater than either alone.
Interestingly, amyloid plaques were reduced throughout a much greater portion of the brain with combined GENUS, as it elicited a unique microglia response that extended to the medial prefrontal cortex, a region that could not be reached with one week of visual or auditory stimulation alone.
A key question now is to understand why these reactions were only observed when stimuli were triggered at a 40 Hz, as mice exposed to different frequencies and pulse patterns did not display better results than the control group.
What’s even more interesting is that research has shown that neurons can encode stimuli without synchronizing their firing rate to the stimulus frequency, a pattern that has also been witnessed in the study. Only some neurons fired at a different rate in reaction to a change of auditory GENUS frequency but they were too few to generalize this observation. The population of neurons as a whole was unresponsive to variations in GENUS frequency. This means that the observed changes in vasculature, amyloid and tau protein level and behaviour cannot be explained by overall changes in firing rate.
While waiting to find the reason for this phenomenon, Tsai and her team have already tested combined GENUS in healthy volunteers to assess its safety. They are now recruiting early-stage Alzheimer’s disease patients to determine whether the results observed in mice can be replicated in humans.