Breast cancer is one of the most common cancers among women, and almost 30% of primary breast tumours metastasize to other organs, with bone among the most frequent metastatic site. To understand why breast cancer spreads to bone, researchers at Indiana University Purdue University Indianapolis (IUPUI) are studying the mechanics of cell migration. Their goal is to explain how cancer cells generate enough force to move from the primary tumour site through the body and then settle in bones (Sci. Reports 10.1038/s41598-019-42132-x).
“From a physics point of view, all the cell migration is driven by mechanical force,” explains Jing Liu, head of the Bioimaging and Biophysics Lab at IUPUI. “We really want to discover the force architecture of a cell and deliver the biomechanical and biophysical explanations toward cellular activities. The major focus of our lab is developing imaging methods to physically interpret cancer biology. We are working with mathematicians and engineers to develop a mathematical model and physical model of the cell migration.”
Liu and colleagues employed a Förster resonance energy transfer (FRET)-based molecular tension sensor to monitor the force dynamics during cell movement. The sensor acts like a spring to measure the tiny amount of force that’s generated by the cancer cell through focal adhesion and which drives the cell to move. As the cancer cell moves, the spring expands. The researchers can then measure the force by monitoring the change of FRET interactions.
To evaluate the focal adhesion forces of tumour cells interacting with osteocytes (mechanosensitive bone cells), the researchers conducted FRET analysis on breast cancer cells transfected with a vinculin tension sensor. They analysed the tumour cells’ migratory behaviour using real-time live cell imaging.
They found that treating tumour cells with osteocyte-conditioned media decreased the tensile forces in their focal adhesions and decreased their migratory potential. Conversely, tumour cells treated with media derived from bone cells exposed to mechanical stimulation exhibited increased tensile forces and migratory potential.
These findings suggest that osteocytes play a critical role in modulating the migratory behaviour of tumour cells, acting as both a stimulator and an inhibitor, depending upon the biophysical condition of the bone microenvironment.
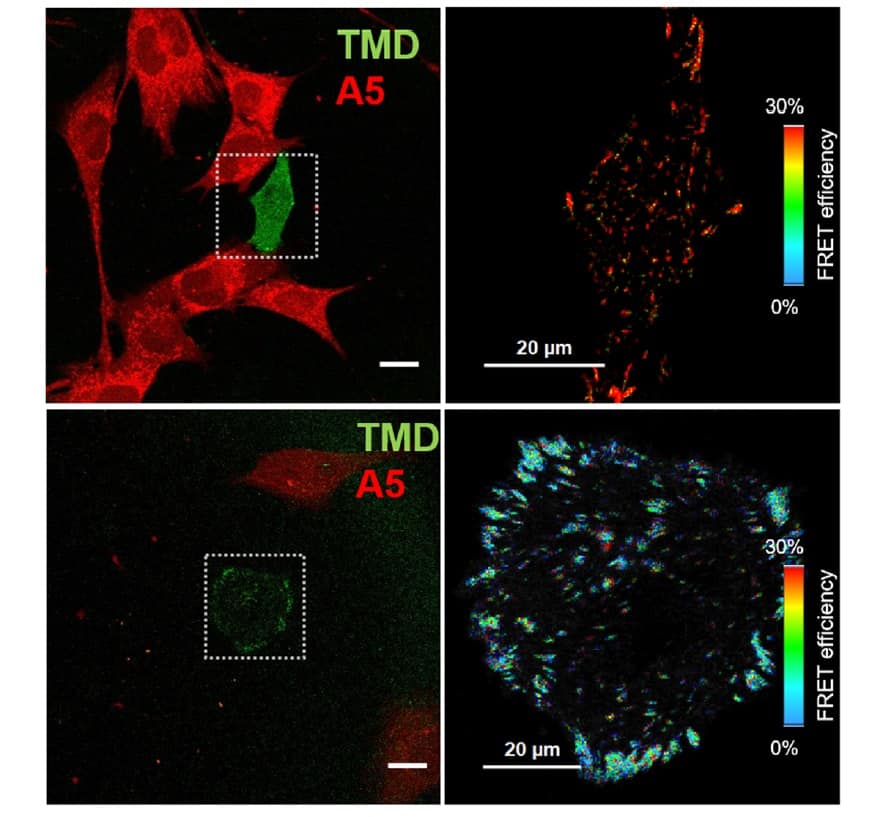
The IUPUI team also observed that focal adhesion tension in individual tumour cells was affected by the distance from bone cells when the two cells were co-cultured: tumour cells close to bone cells exhibited lower tension and decreased cell motility.
The researchers hope that these findings might lead to clues for how to control — and eventually stop — cell migration. “This [technique] gives us a more precise measurement of how fast the cell is moving and where the cell will go to,” says Liu. It will also provide feedback to cancer biologists, showing the impact of a drug or other treatment on the movement of the cells.
“The basic idea is to use imaging as a method to see some of the physical parameters in cancer biology,” Liu says. “Instead of only being able to look at millions of cells at time, technology has enabled us to examine a single cell. When the system is going smaller and smaller, the physical parameters inside the biological system become more and more useful and more and more important.”
The authors conclude that their results might contribute “not only to our basic understanding of tumour growth and migration in the bone microenvironment, but also toward developing novel therapies to prevent bone metastasis associated with breast cancer”.