Trace amounts of salt can help control the “coffee-ring effect” that occurs when a solvent evaporates from a solution containing non-volatile particles. The technique, which was developed by researchers in China, works for a variety of substrates, including graphene, graphite and polymers, and could help engineers deposit more uniform coatings and dyes.
Although the coffee-ring effect gets its name from the familiar stain left behind when coffee drips down the side of a cup and spreads around the base, it occurs in many other liquids, too. In this well-studied process, the edges of a drop of liquid on a surface become pinned to that surface, preventing the drop from shrinking as the liquid evaporates. Instead, the drop flattens out, pushing the liquid – and anything suspended in it – to the edges. By the time the drop evaporates completely, most of the suspended particles have reached the edges and remain behind as a dark ring.
As well as being an unsightly nuisance on your desk or table, the coffee-ring effect is the bane of many an industrial process (including printing, coating, dyeing, complex assembly and micro- and nanofabrication) because the ring structures it produces are so non-uniform. Researchers have explored many strategies for avoiding the effect, from designing paints and inks that produce an even coating when they evaporate to changing the shape of the suspended particles. Various groups have also tried adding surfactants, polymers, sol-gel inducers, co-solvents and even proteins to the solutions, while others have experimented with using external optical and electrical fields to modify the ring-forming process.
A new and efficient technique
A team of researchers led by Haiping Fang of East China University of Science and Technology and Guosheng Shi of Shanghai University has now developed a new and efficient technique that involves adding trace amounts of various salts to the solution. When the researchers tested their technique on a solution containing dyes placed on graphene, polymers and other substrates that contain chemical structures known as aromatic rings, they found that the colour of the dye remained even across the substrates. Molecular dynamics simulations revealed that strong interactions (known as cation-π interactions) between the hydrated cations in the solution and the aromatic rings in the substrates work to inhibit the coffee-ring effect, since they promote a more uniform adsorption of suspended matter onto the substrates.
In their experiments, Fang, Shi and colleagues created solutions with different concentrations of sodium chloride (NaCl) by mixing the salt into aqueous suspensions of polystyrene microspheres. They then placed drops of the salty microsphere mixtures onto a graphene substrate grown via chemical vapour deposition. As a control, they tested solutions without NaCl. In separate experiments, they also tried the technique on substrates such as glass, which do not contain aromatic rings.
No ring effect with 8.0 mM NaCl
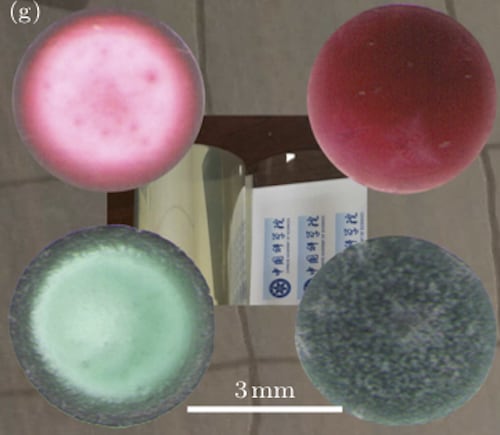
After allowing the drops to evaporate at around 10° C, the researchers used optical microscopes and greyscale analysis to study the dried patterns left on the substrates. In suspensions without NaCl, they observed ring-like patterns with a dark rim and a light grey centre on the graphene. For the salty suspensions, however, the rings were absent, and the image contrast between the rim and the centre of the pattern gradually diminished for mixtures with increasing NaCl concentrations. At a concentration of 8.0 mM NaCl, the pattern appeared completely uniform.
The team, who report their work in Chinese Physics Letters, found the same effect with other salts such as lithium chloride (LiCl), potassium chloride (KCl), calcium chloride (CaCl2) and magnesium chloride (MgCl2) at different concentrations. They also observed similar behaviour with other aromatic-ring substrates, including natural graphite and a common thermoplastic resin called polyethylene terephthalate (PET). When the experiments were repeated with glass substrates, however, the coffee-ring effect lingered despite the addition of salt, confirming the importance of the hydrated cation-π interactions with aromatic rings.
Cation-π interactions remain strong
Cation-π interactions are known to play crucial roles in the structure, dynamic processes and functions of both living and non-living systems. Until recently, researchers believed that these interactions weakened when the cations were hydrated, so they usually neglected them. Earlier work by Fang and Shi’s team, however, suggested that this approach was incorrect. “Our previous theoretical and experimental studies showed that these interactions remain strong enough to result in the strong adsorption of hydrated cations on graphitic surfaces (such as carbon nanotubes, graphene, graphite and graphene oxide),” Fang explains. “This is because the polycyclic aromatic ring structures of these materials themselves include more π electrons.”
Shi notes that since the cation-π interaction also exists between other cations and aromatic rings, metal ions such as Fe2+, Co2+, Cu2+, Cd2+, Cr2+ and Pb2+ might prove just as effective as salt for controlling the coffee-ring effect. The team’s future studies will focus on choosing the best cations for different applications, he tells Physics World.



