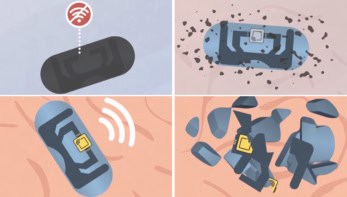
Temporary cardiac pacemakers provide essential pacing for patients with short-term heart rhythm disorders following cardiac surgery or while waiting for a permanent pacemaker. Such devices typically require external hardware with leads inserted through the skin, risking infection and limiting patient mobility. In addition, when the implanted device is no longer needed, its removal risks damage to heart tissue.
To overcome these limitations, researchers in the US have devised an implantable cardiac pacemaker that operates without external leads and completely dissolves in the body after completion of therapy. The device is made from materials that fully resorb through natural biological processes in a time-controlled manner, eliminating the need for surgical removal.
Such a bioresorbable, leadless, cardiac pacemaker could provide postoperative control of heart rate and rhythm without the risks associated with traditional temporary pacing systems. The research team – headed up at Northwestern University and The George Washington University – describe the innovative device in Nature Biotechnology.
Device design
To eliminate the need for batteries and thus miniaturize the device, the researchers used wireless energy transfer to deliver power and control commands to the pacemaker. The device’s power-harvesting receiver comprises an inductive coil made from tungsten-coated magnesium (W/Mg), a radiofrequency PIN diode based on a silicon nanomembrane, and a dielectric interlayer of the biodegradable polymer PLGA. The pacemaker also includes flexible W/Mg extension electrodes connected to dissolvable metallic contact pads, which attach to the myocardium to deliver the electrical stimuli.
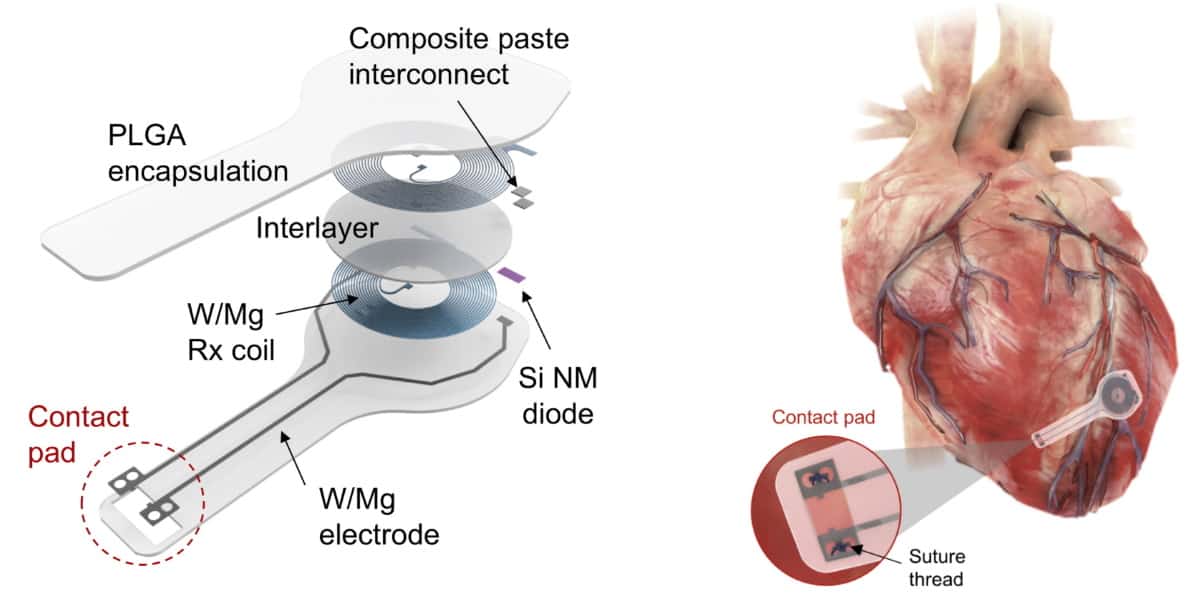
The entire system is just 16 mm wide and 250 μm thick, and made entirely of bioresorbable materials: PLGA dissolves into its monomers, glycolic and lactic acid, while W/Mg and the silicon nanomembrane degrade into nontoxic products. The flexibility of these materials also enables the device to conform to the curved surface of the heart and adapt to its movements.
“Significant advances in materials science and organ conformal bioelectronics made this work possible,” explains Igor Efimov, who led the study along with John Rogers and Rishi Arora. “Wireless battery-free pacemakers were developed a while ago. But they suffered from mechanical mismatch between the rigid device and soft tissue of a beating heart, which resulted in high mortality of animals and was not easily translatable to humans.”
“The development of soft organ-conformal electronics by Rogers’ group solved this problem,” says Efimov. “The next step was the development of bioresorbable electronics. This was a game changer for many clinical needs of temporary implantable devices.”
Proof of pacing performance
The team first tested the pacemaker on ex vivo mouse and rabbit hearts, using RF power from a nearby transmitting antenna to initiate pacing. Tests in slice of human cardiac tissue also demonstrated successful pacing.
Next, the group used the pacemaker to treat AV block (impaired signal transmission from the atria to the ventricles) in an ex vivo mouse model. Electrocardiogram (ECG) recordings and measurements of optical action potentials confirmed that the device could deliver effective ventricular pacing, driving the rhythm of the heart to treat the AV block.
The researchers also demonstrated in vivo pacing in an adult dog during open-chest surgery. They recorded a maximum pacing distance (between skin and transmission coil) of 17 cm, validating the pacemaker’s capability for long-range wireless energy transfer. These findings suggest that the device can achieve the necessary power transfer for operation in adult human patients.
Lastly, the team surgically implanted biodegradable pacemakers in rats and performed daily pacing trials on awake or lightly sedated animals. Changes in the ECG signal indicated successful ventricular capture. The device successfully performed pacing for four days after implantation, then its performance started to degrade until after six days the pacing failed. The timescales for stable operation and complete bioresorption can be tailored to meet specific clinical requirements. “Devices can be coated with materials that have a slow rate of resorption, which would ‘program’ the lifetime before the device is resorbed,” explains Efimov.
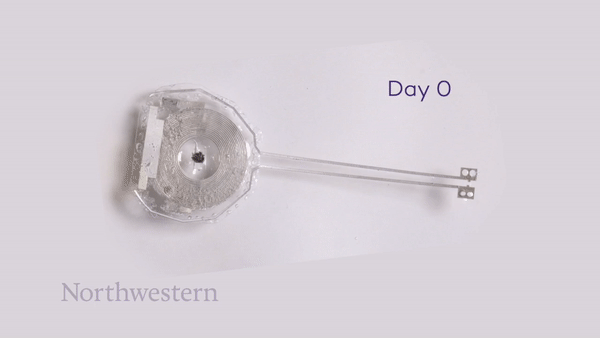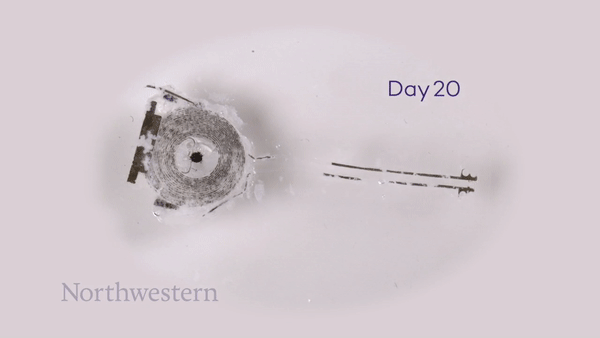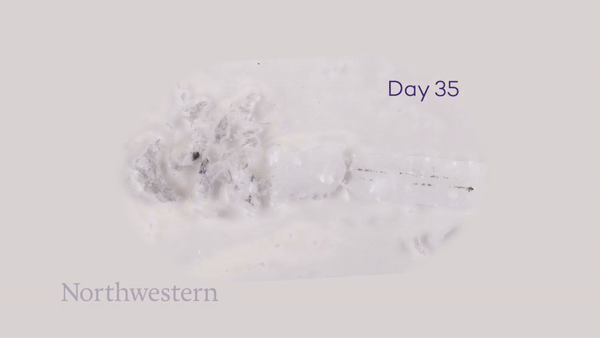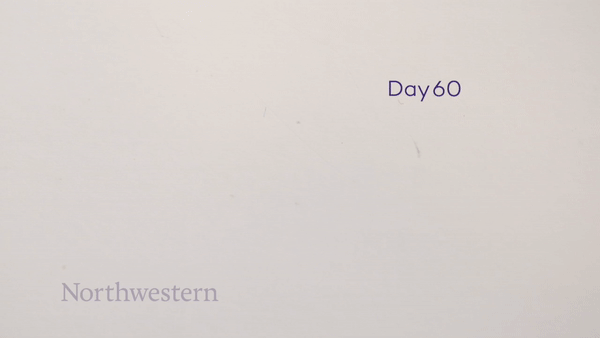
To monitor the bioresorption process, the researchers recorded CT images of the rats over seven weeks. One week after implantation, the pacemaker maintained its shape and contact with the heart. The device then shrank and collapsed over time, largely dissolving within three weeks, with the remaining residues completely disappeared after 12 weeks. They note that the implantation and resorption of the bioresorbable pacemaker did not impact the rats’ natural physiology.

Bridging the gap between biology and electronics
The researchers are now planning to develop bioresorbable pacemakers for paediatric patients with AV block following septal defect repair, who require a temporary pacemaker for several days after surgery. They will also design devices for adult patients who also frequently experience temporary AV block after valve repair.
“A similar approach can used for many other cardiac, neural and muscle applications,” Efimov tells Physics World. “We also believe that this approach can be used to design a temporary defibrillator to treat post-operative atrial fibrillation, which occurs in 30% of CABG [coronary artery bypass graft] patients and after other cardiac surgeries.”