For the first time ever, researchers have delivered an antibody therapy drug directly into the brain to target breast cancer metastases. A team at Sunnybrook Health Sciences Centre in Toronto used MR-guided focused ultrasound (MRgFUS) to non-invasively and temporarily open the blood–brain barrier (BBB), enabling the monoclonal antibody trastuzumab to reach specific areas of the brain. Writing in Science Translational Medicine, the researchers describe the first visual confirmation that focused ultrasound can improve delivery of targeted antibody therapy across the BBB.
The BBB consists of a thin layer of cells that protect the brain from toxins, viruses and bacteria, but which also blocks the delivery of therapeutic drugs. Trastuzumab, an antibody therapy that helps the immune system fight cancer cells, is used in conjunction with chemotherapy and radiation therapy to treat breast cancer metastases in the brain. However, it is 100 times larger than typical compounds able to enter the brain across the BBB.
This first-in-human trial suggests that MRgFUS provides a safe and effective method to deliver drugs across the BBB. It sets the stage for the possibility of delivering both established and novel therapies for numerous brain conditions that otherwise cannot gain access to the brain.
Early findings
Principal investigator Nir Lipsman, director of Sunnybrook Research Institute’s Harquail Centre for Neuromodulation, and colleagues report the results from the first four patients participating in the ongoing Phase I clinical trial. All patients had Her2-positive breast cancer with brain metastases, and had previously received whole-brain radiation and/or stereotactic radiosurgery, plus chemotherapy, to treat progressive intracranial and stable systemic disease.
To perform BBB opening, the team used Insightec’s ExAblate, a helmet-like focused ultrasound device containing over 1000 transducers that converge ultrasound waves onto discrete points in the brain. The pulsed FUS was delivered in conjunction with a continuous intravenous infusion of microbubble ultrasound contrast agent and trastuzumab-based therapy.
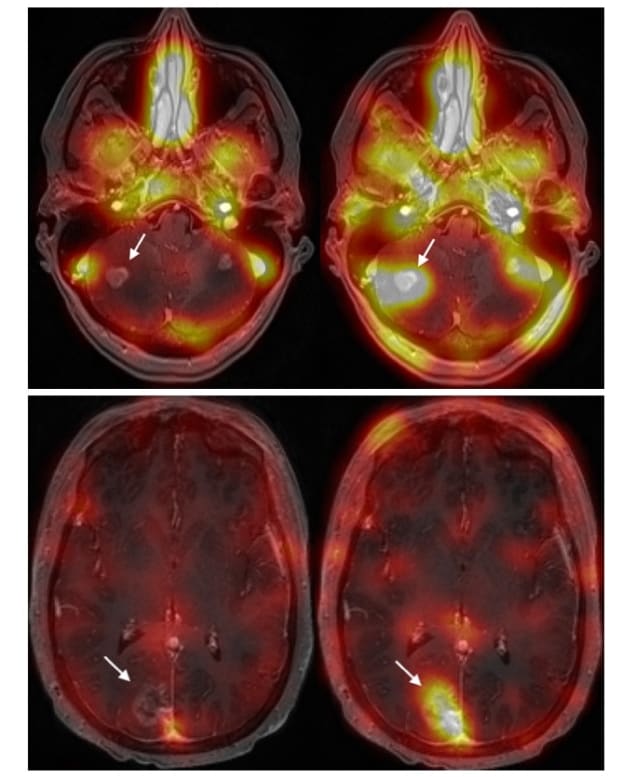
The trastuzumab used in the study, developed by Raymond Reilly from the University of Toronto and colleagues, was radiolabelled with 111In. Use of this radiotracer-infused trastuzumab allows direct visualization of trastuzumab distribution in single photon emission computed tomography (SPECT) images.
The team delivered a total of 20 outpatient treatments combining transcranial MRgFUS with standard of-care trastuzumab injection, with an average treatment time of 138 min. Patients had a SPECT scan prior to treatment, 4 hr after treatment and 48 hr later. The researchers performed additional SPECT scans 30 and 90 days following the final treatment and continue to monitor the patients.
The SPECT images showed trastuzumab precisely targeting the tumours. After BBB opening, the researchers could directly visualize increased SPECT signal within the sonication volume for all lesions treated. MR images revealed that the patient’s brain tumours decreased in size by between 7% and 31%, at up to four months after treatment. The procedure caused no serious adverse effects. Lipsman reports that three of the patients remain stable, and that one patient died from progressive, non-intracranial systemic disease.
The researchers note that one potential advantage of MRgFUS over other physical and biological approaches to brain drug delivery is the high degree of spatial and temporal control that it provides. MRgFUS enables selective targeting of single or multiple brain lesions, which can be located at the extremes of the brain or in deep eloquent central regions of the cortex that control bodily function.
“This study adds the brainstem, cranial nerve nuclei and cerebellum to the list of regions that can be safely and precisely targeted with MRgFUS, all areas in which radiation and surgery may be limited,” the authors write. Also, because MRgFUS can be combined with diverse therapeutic agents, therapies with less side effects can be used, improving treatment tolerability and patient safety.
Future outlook
This study is continuing to enrol patients, with the goal of performing a 10-patient trial. “Completing our current trial is an important first step,” Lipsman tells Physics World. “It will help inform what the design of a larger trial would look like, what kinds of outcomes we should be measuring, and how to optimize a study for safety and feasibility. The goal is to determine what role focused ultrasound will play in the management of brain cancer patients. For that, creating partnership and collaborations with other institutions will be key.”
Sunnybrook is a Focused Ultrasound Center of Excellence, the only Canadian site recognized as such by the Focused Ultrasound Foundation, which is helping to fund this study. Lipsman says the team is investigating several aspects of using focused ultrasound in brain cancer.
Focused ultrasound tackles brain tumours in a myriad ways
“We want to determine whether peripheral biomarkers, or so-called liquid biopsy, can be enhanced following BBB opening, to help with less invasive approaches to diagnosis and treatment monitoring,” he explains. “We also will conduct more comprehensive tests looking at the safety of BBB opening, including what impact, if any, we are having on important inflammatory markers and other features of the BBB.”
“Importantly, we are interested also in streamlining the procedures, making them faster, more efficient and more comfortable for patients,” he adds. “Enhancements in imaging technology will aid in targeting, and improvements in hardware and software will give us even better control of microbubbles and ultrasound transducers, to control just how much of the BBB is open and hence how much of a therapeutic can be delivered. All of these advancements are underway and will be rolled out in future trials that are now under active development.”