Energy-saving solid-state lighting and full colour displays have come along in leaps and bounds in recent years with materials that emit bright, pure colours. To create white light, we need red, green and blue sources, but blue light is the most challenging to produce. Indeed, it took researchers an extra two decades to create the first blue-light emitting diodes (LEDs) after red and green ones were made in the 1950s and 60s.
Efficient blue-light emitting materials for displays need not only to emit bright light, they also need to do this over a narrow wavelength range. Making such materials has proved to be no easy task, and even the purest and defect-free candidates, such as epitaxially-grown gallium nitride films, only manage to reach a maximum photoluminescence quantum yield (PLQY) of less than 1%. This low value is due to rapid non-radiative recombination of charge carriers (electrons and holes) through surface and bulk defects (or traps) and a low radiative recombination rate associated with a small exciton (electron-hole pair) binding energy.
Although other materials, such as inorganic phosphors, fare much better when it comes to PLQYs, their insulating properties result in high turn-on voltages, which means that they cannot be used in LEDs. They unfortunately also emit spectrally broad light.
2D layered perovskites
Organic-inorganic hybrid perovskites could be the answer here. These materials are one of the most promising thin-film photovoltaics around today thanks to the fact that they can absorb light over a broad range of solar-spectrum wavelengths. Researchers led by Edward Sargent of the University of Toronto in Canada have been studying 2D layered perovskites with the composition R2PbBr4, where R stands for organic ammonium cations, Pb is lead and Br is bromine. Such perovskites boast fast radiative recombination rates (thanks to localized excitons) and a narrow light emission linewidth.
“In particular, we investigated the effect of electron-phonon interactions on the luminescence of single crystals of 2D perovskites,” explains team member and lead author of the study Xiwen Gong. “We found that reducing these interactions can lead to bright blue emission in these materials.” Phonons are quantized lattice vibrations that behave like particles.
The researchers studied the strength of electron-phonon coupling and how rigid the crystals were using several techniques, including deformation potential analysis, resonance Raman spectroscopy, single-crystal X-ray diffraction, neutron scattering and solid state nuclear magnetic resonance. The neutron studies were carried out at Oak Ridge National Laboratory.
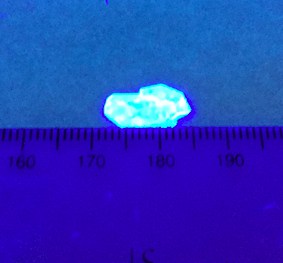
PLQY of up to 79% and a linewidth of just 20 nm
The results reveal that the brightest emitters are those that are the most rigid. “By varying the molecular configuration of the ligands on the perovskite, we found that we can reach a PLQY of up to 79% and a linewidth of just 20 nm by controlling the crystal rigidity and electron-phonon interactions,” says Gong.
“The most rigid structure suppresses dynamic vibrations in the 2D perovskite crystals, which leads to decreased electron-phonon interactions,” Sargent tells Physics World. “This slows the electron band-edge to trap transition process, thereby increasing the brightness of the 2D perovskite emitter.
“Designing crystals structures with electron-phonon interactions in mind thus provides us with a previously unexplored way to improve the properties of optoelectronic materials,” he adds.
Barry Rand of Princeton University in the US says that the new work is an “impressive step forward. It shows that this class of layered perovskites has the capacity to host very high efficiency blue emission, a necessary milestone in their use in efficient LEDs. This is especially important as blue LEDs have lagged considerably behind the performance of red and green emitting devices.”
Sargent and colleagues, reporting their research in Nature Materials , worked with large single crystals with dimensions of millimetres in this work. They say they now plan to move to 100-nm-thick active materials so that they can indeed incorporate them into LED devices.