
Researchers from the University of Wisconsin-Madison, along with colleagues from Sweden and the UK, have analysed, for the first time, the bacterial genome composition (microbiome) of the gut of individuals diagnosed with Alzheimer’s disease (AD). The authors reported a decrease in microbial diversity and a difference in bacterial composition for individuals with AD, compared with healthy subjects. This difference in composition correlated with the extent of AD pathology and reflected a functional alteration of AD-associated bacteria. These findings hint to the potential of developing therapies to target gut bacteria (Sci. Rep. 7 13537).
The last decade has seen an exponential increase in the number of publications that associate a gut microbial alteration – known as “dysbiosis” – with diseases such as obesity, diabetes, irritable bowel syndrome and Parkinson’s disease.
AD is a neurodegenerative disease characterized by the accumulation of amyloid-β plaques and neurofibrillary tangles (aggregates of hyperphosphorylated tau protein) in the brain and is estimated to affect 46 million people worldwide (World Alzheimer Report 2015). Despite a wealth of research, there isn’t a complete understanding of the mechanisms behind the onset and development of this disease.
The gut microbiome in AD
The “microbiome” refers to the collection of genetic material from a community of microorganisms. Recent studies, using AD mouse models, reported that changes in gut microbiome influenced the accumulation of amyloid-β (see, for example, Sci. Rep. 6 30028 and Sci. Rep. 7 41802).
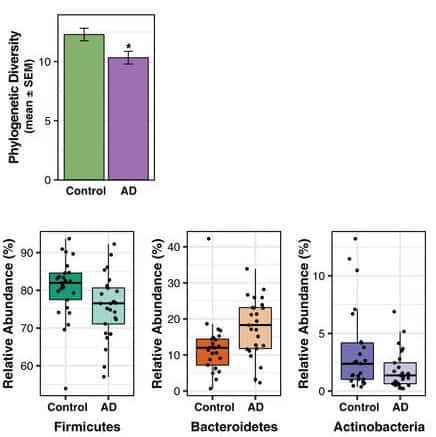
To fully characterize the gut microbiome of individuals with AD, the authors performed bacterial gene sequencing on faecal samples collected from 50 participants matched for age, sex, diabetes and weight: 25 with diagnosed AD and 25 healthy controls. Participants with AD displayed a less rich gut microbiome and, in particular, they showed a decrease in abundance of Firmicutes and Actinobacteria, and an increase in Bacteroidetes, compared with the healthy participants.
The gut-brain axis
The authors suggested that the observed dysbiosis supports the hypothesis that gut microbiome alterations may promote AD pathology via the activation of the immune system and the development of insulin resistance. They proposed that, since Actinobacteria have anti-inflammatory properties and Bacteriodetes trigger pro-inflammatory responses, the opposing changes in abundance of these bacteria can stimulate inflammation. Additionally, they cite previous research that linked a decrease in Firmicutes abundance to diabetes incidence and the latter to an increase in amyloid-β accumulation.
To further support this hypothesis, the authors studied the relationship between the levels of AD biomarkers (biological molecules used as pathology indicators) measured from cerebrospinal fluid and the gut bacterial abundances of AD participants – and found them to be correlated. This result linked a more pronounced dysbiosis to a greater AD pathology.
This work adds to the growing evidence of a bidirectional interaction between the digestive tract and the brain via the nervous, endocrine and immune systems – the gut-brain axis. Future research to study the influence of gut microbiome in brain activity and progression of a disease, such as AD, may lead to the development of new targeted therapies.



