Researchers at the University of Cambridge in the UK have developed a new type of neural implant made from pluripotent stem cells grafted into flexible electrode arrays. The device, which greatly improves the tissue–electronics interface, could be used to drive advanced neuroprosthetic limbs to a degree not currently seen in the clinic and improve function in paralysed limbs by bypassing injuries to the nervous system.
Creating functional neural implants is difficult since scar tissue tends to form around the electrodes over time, degrading the connection between the implant and the nerve.
To address this problem, the researchers, co-led by Damiano Barone, combined two advanced therapies: regenerative medicine, in which cells are introduced into the body to repair native tissue; and bioelectronics, where devices are implanted to interface with and control tissue. By including a layer of (skeletal muscle) cells reprogrammed from induced pluripotent stem cells (cells that can be differentiated into any type of cell) between the electrodes and the tissue, they found that the nerve on which the interface was implanted was able to grow into and biologically connect with the interface.
“This strategy allowed us to achieve a much higher quality and quantity of connections with nerves and prevent scar tissue,” explains Barone. “These connections can in turn be used to drive a muscle that is disconnected from the brain because of injury, or a prosthesis, so restoring mobility.”
Completely new way of interfacing with the nervous system
This is the first time that induced pluripotent stem cells have been used in a living organism in this way, and the concept opens the door to a completely new way of interfacing with the nervous system, Barone adds.
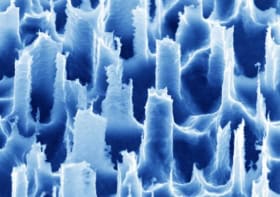
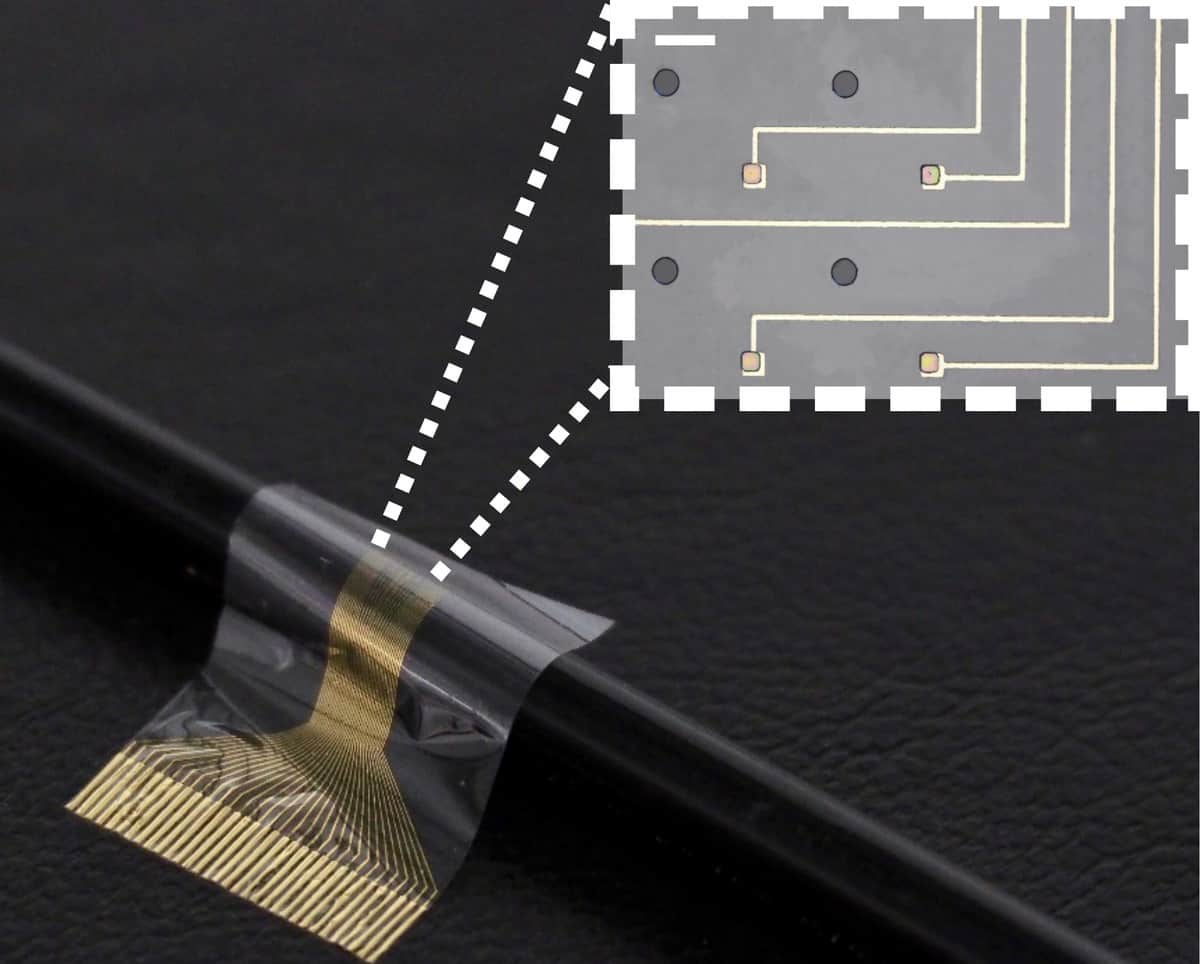
To create their biohybrid device, the Cambridge researchers began by fabricating a thin-film electrode array and decontaminating it. Next, they seeded induced pluripotent stem cells onto the device and induced these cells to transform into muscle cells.
“Our device is highly flexible and biocompatible and has high-resolution recording and stimulating capabilities,” Barone tells Physics World. “The stem cells we used in this project have the particular advantage of becoming the required type of cell in just eight to 10 days with a very high density.”
The team tested its device by implanting it into the paralysed forearms of rats. The muscle cells integrated with the nerves in the rats’ limbs and while movement was not restored, the device was able to detect signals from the brain that control movement, which is an important advance. The cells survived for the duration of the experiment (28 days), which is longest time period ever achieved for such a trial.

Brain–computer interfaces: tailoring neurotechnology to improve patients’ lives
As well as being easy to integrate into tissue, and the fact that it is stable over the long term, the device could be implanted using keyhole surgery given its small size. This is in contrast with other neural interfacing technologies that are complex and much larger.
The researchers, who report their work in Science Advances, now plan to connect the biohybrid interface to either prosthetics or paralysed limbs to improve mobility in animal models and eventually in humans. “We also hope to explore the concept of biohybrid interfaces in other areas of clinical need with different electronic designs and different cell types,” says Barone.