PET scanners use time-of-flight (TOF) technology to reduce image noise and improve the identification of cancerous lesions. TOF works by using the time difference between detection of the two PET annihilation photons to more accurately localize the annihilation event. However, many current clinical PET scanners do not have TOF capability, and miss out on the improved diagnostic confidence that it confers.
“There is a significant cost difference between TOF and non-TOF PET scanners because of the high cost of the scintillator used for TOF,” says Daniel McGowan from the University of Oxford and Oxford University Hospitals NHS Foundation Trust, noting that one of GE Healthcare’s most successful product lines is a non-TOF PET scanner, the Discovery IQ. “We estimate that approximately one in three PET/CT sites in the world currently do not have access to TOF technology.”
To level this playing field, McGowan and collaborators are employing deep learning to bring the benefits of TOF to PET images reconstructed without TOF information. Writing in the European Journal of Nuclear Medicine and Molecular Imaging, they describe their proposed deep learning for TOF image enhancement (DL-TOF) approach.

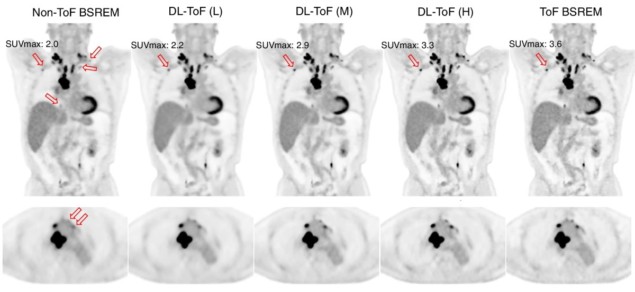
The team developed three DL-TOF models (based on U-Net convolutional neural networks) to transform non-TOF PET data into corresponding TOF-like images. The models employed different levels of TOF strength (low, medium or high) to trade off contrast enhancement against noise reduction.
The researchers note that the neural network does not add TOF information to the PET coincidence data, but rather, it learns how TOF information alters image characteristics and then replicates these changes in non-TOF input images. “This is exactly the kind of task that deep learning algorithms do very well,” McGowan explains. “They can find patterns in the data and create the transformation that produces visually attractive and quantitatively accurate images that give high diagnostic confidence to the reporting radiologist or physician.”
Model evaluation
To train, validate and test the models, the team used PET data from 273 whole-body FDG-PET oncology exams performed at six clinical sites with TOF-capable PET/CT scanners. The PET data were reconstructed using the block-sequential-regularized-expectation–maximization (BSREM) algorithm, with and without TOF.
After training, the researchers evaluated the model performance using a testing set of 50 images. They examined standardized uptake values (SUVs) in 139 lesions and normal regions of liver and lungs, using up to five small lesions and five volumes-of-interest in the lungs and liver per subject.
Comparing the outputs of the three DL-TOF models with the input non-TOF images showed that the models improved the overall image quality, reducing noise and increasing lesion contrast. In the original non-TOF image, the lesion SUVmax differed from the target TOF image by −28%. Applying the DL-TOF low, medium and high models resulted in differences of −28%, −8% and 1.7%, respectively. The models also reduced differences in SUVmean from 7.7% to less than 2% in the lungs, and from 4.3% to below 1% in the liver.
Diagnostic application
In addition to the quantitative evaluation, three radiologists independently rated the testing set images in terms of lesion detectability, diagnostic confidence and image noise/quality. Images were assessed based on a Likert scale, which ranges from 0 (non-diagnostic) to 5 (excellent).
The DL-TOF high model significantly improved lesion detectability, achieving the highest score of the three models. In terms of diagnostic confidence, DL-TOF medium achieved the best score, while DL-TOF low scored the best for image noise/quality. In all cases, the top-performing model outscored the target TOF image. These results highlight how the DL-TOF model can be tailored to balance lesion detection versus noise reduction, according to the preference of the image reader.
“Overall, in terms of diagnostic confidence, the DL-TOF medium model provides a better trade-off in our test set, as a lower noise and improved detectability are desirable features for an image reconstruction or enhancement technique,” the team writes.

Neural networks improve PET time-of-flight estimates
Finally, the researchers applied the DL-TOF models to 10 exams acquired on a non-TOF PET scanner, to illustrate the generalizability of the trained models. While there was no ground truth or target image for comparison, visual inspection showed that the images were free of obvious artefacts and exhibited the expected image enhancement. These findings suggest that the models may work on data from scanners that were not part of the algorithm training dataset.
McGowan notes that this initial work focused on whole-body FDG-PET for oncology as this is the main clinical application of PET today. “However, with the advent of new tracers and increased interest in organ-specific imaging, we are currently testing the existing algorithm in the context of these new applications, which were not represented in the training data, and deciding whether additional training is needed to achieve adequate performance for other indications,” he tells Physics World.
![]() AI in Medical Physics Week is supported by Sun Nuclear, a manufacturer of patient safety solutions for radiation therapy and diagnostic imaging centres. Visit www.sunnuclear.com to find out more.
AI in Medical Physics Week is supported by Sun Nuclear, a manufacturer of patient safety solutions for radiation therapy and diagnostic imaging centres. Visit www.sunnuclear.com to find out more.



