
Bacterial therapies, in which living bacteria are used to deliver drugs or other payloads to kill cancerous cells, could provide an alternative treatment for a wide range of cancers. When bacteria infiltrate the human body, the immune system triggers a fighting mechanism against the foreign substance, with the aftermath of such events dependent on the potency of the bacterium. However, some probiotic bacteria, such as Escherichia coli Nissle 1917 (EcN), easily resist the immune system’s lines of defence. This could be problematic if such bacteria are being considered for therapeutic applications.
Living bacteria can be engineered to kick back against the immune system, resulting in two potential outcomes: a compromise in the immune system after bacteria delivery; and the living bacteria causing toxicity to its host cells. Over the past decade, researchers have explored the reduction of toxicities from live bacteria by genetically deleting the parts of the bacterium that can cause toxicity; but this can lead to unwanted mutations in the bacterium itself and may substantially decrease therapeutic efficacy.
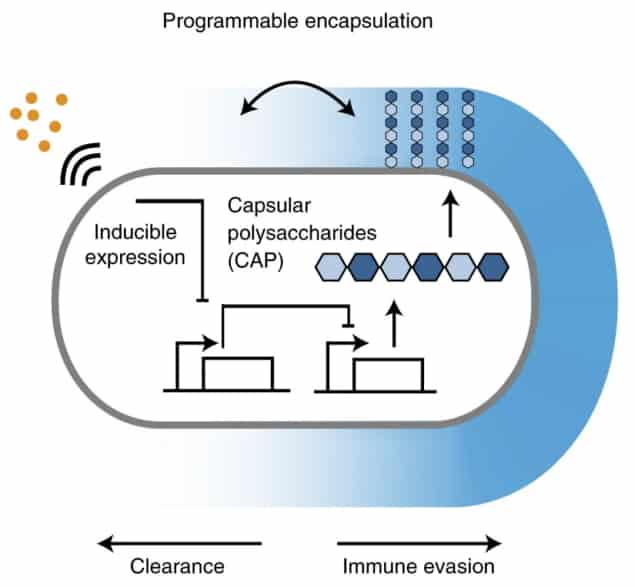
A team of engineers from Columbia University has now determined an effective approach to enhance the delivery of living engineered bacteria into cells, while maintaining the bacterium’s integrity and minimizing toxicity. Reporting their findings in Nature Biotechnology, the researchers describe a way of coating engineered bacteria with an inducible capsular polysaccharide (iCAP) that responds in a smart manner when delivered into the body.
Capsular polysaccharide (CAP) is a layer of water molecules that coats the surface of natural bacteria and acts as a shield against foreign infections. By converting CAP into iCAP, the researchers could apply programmable external stimulus that enables the engineered bacteria to evade immune attack, survive for a considerable duration in the host environment and deliver a tolerable therapeutic dose.
Guiding the bacteria
Cancer cells possess a natural ability to evade the immune system, which is one of the significant hallmarks of cancer. Since engineered bacteria are also required to evade immune attack, targeting bacteria to tumours becomes a herculean task, requiring a highly sophisticated design to enable adequate localization of the bacteria in the tumours.
The researchers leveraged synthetic gene circuits to dynamically control how the bacteria interact with their surrounding environment using the iCAP. As well as protecting against environmental pressures and forming a barrier for the bacteria wall, CAP has also been reported to play important roles in sensing immune responses. To control CAP expression, the authors introduced a small-molecule inducer termed IPTG. Induction of the CAP with IPTG modulated the bacteria’s interactions with circulating antimicrobials, bacteriophages, acids and the host immune system.
The iCAP system for cancer applications
While bacterial therapies for cancer continue to advance, developing a robust system for killing all tumours might seem insurmountable. As a starting point, however, the researchers demonstrated that the iCAP system can control the therapeutic delivery in mouse models.
To investigate the efficacy of iCAP, the researchers first examined the bacterial viability in human whole blood. They found that the engineered bacteria survived significantly longer than bacteria with natural CAP. Furthermore, after administering mice with iCAP bacteria, they observed lower inflammatory responses compared with the non-engineered bacteria.
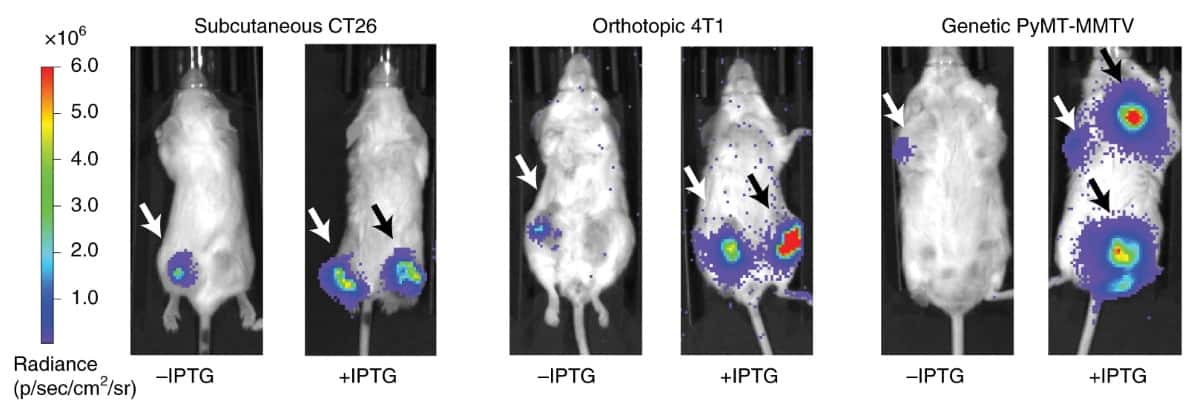
In tumour-bearing mice, iCAP also enabled the translocation of therapeutic bacteria to multiple distal tumours throughout the body, with increased trafficking compared with natural bacteria. Additionally, delivering an EcN iCAP construct engineered to produce an anti-tumour toxin led to a reduction in tumour growth in the mice, demonstrating its therapeutic efficacy.
Tal Danino, senior author of this study, now plans to further explore the use of iCAP and other bacterial-based therapeutics to accelerate clinical translation in the future.



