Physicists are developing better oils and lubricants that promise to improve the fuel efficiency of cars and reduce greenhouse-gas emissions.
Lubricants are essential in modern life. Car engines and gearboxes run smoothly thanks to sophisticated oils and greases, while computer hard disks rely on thin organic films to ensure that the “read/write” head can move reliably at high speeds across the recording medium. According to some analysts, however, the direct costs of friction and wear can account for nearly 10% of the gross national product (GNP) in many industrial nations. Moreover, they estimate that cost savings of up to 1% of the GNP could be achieved simply by using the right lubricant for the job.
Lubricants are remarkable fluids. During winter in Detroit, for example, the same car-engine oil has to operate reliably over temperatures ranging from -40 °C to above 250 °C – the temperature near the top piston ring. It also has to cope with pressures between 105 and 109 pascals, as well as contaminants including metal particles and soot. The final straw is that this fluid must deal reliably with these conditions every day for up to two years – the recommended time between oil changes, according to some vehicle manufacturers.
Surprisingly, one of the major driving forces behind the development of lubricants is the environment. Modern vehicles are required to emit far fewer pollutants than older cars and lorries. Indeed, the emissions from a typical modern vehicle are some 50 times lower than those manufactured in the 1960s.
Carbon dioxide is a natural by-product from the combustion of fuel and is among the most significant pollutants being targeted for reduction. Indeed, vehicles that have a high fuel consumption emit large amounts of carbon dioxide. However, the European Union is taking a strong lead in tackling this problem, and has indicated that the average amount of carbon dioxide emitted by every vehicle should be reduced from today’s average of 200 grammes per kilometre to less than 140 grammes per kilometre from 2008. This is roughly equivalent to improving the average fuel consumption from 33 to 47 miles per gallon. Such an increase would lead to big cuts in terms of carbon dioxide. In the UK alone – where there are roughly 20 million cars, each covering an average of 16 000 km every year – the total annual drop in CO2 would be about 19 million tonnes.
Clearly manufacturers are making a number of engineering changes to their vehicles to try to improve fuel economy. Less well known, however, is the fact that the fuel consumption can be significantly improved just by changing the lubricants. For example, it is possible to decrease the amount of fuel consumed by modern cars by up to 5% simply by switching from a typical multigrade oil to a “friction-modified” lubricant with a lower viscosity. This would lead to an annual CO2 drop of roughly 3 million tonnes in the UK. Remember that this figure is just for the UK and just for cars. Greater CO2 savings are clearly possible if optimized lubricants were also used in trucks and in other machinery.
What is a lubricant?
Car lubricants play four major roles – they control friction and wear in the engine, they protect the engine from rusting, they cool the pistons, and they protect the engine oil stored in the sump from combustion gases.
Some 75%-95% of a typical engine lubricant is made up of a base oil – a mineral oil that has come directly from a refinery. These base oils can naturally contain straight or branched chains of hydrocarbons, hydrocarbon molecules with aromatic rings attached, or these chains can be produced by further chemical reactions of the base oils.
The remainder of the lubricant comprises a variety of additives, which are used to improve performance. Typically these include anti-wear additives, corrosion inhibitors, antioxidants, detergents, dispersants, antifoam additives, and large polymer molecules known as viscosity modifiers, which are added to improve the viscosity variation of the lubricant with temperature.
Indeed, the viscosity is the most significant physical property of a lubricant. The way in which it varies with temperature, shear rate and pressure determines to a great extent how the lubricant performs in an engine. But the chemistry of the lubricant is also important: it must be resistant to oxidation and it must be able to “lay down” a protective film to combat wear and tear where metallic contact is inevitable.
The behaviour of an oil film trapped between two moving surfaces is quantified by the dynamic viscosity, which is measured in millipascal seconds (mPa s). More accurately, the dynamic viscosity relates the shear stress, the shearing force acting on the oil per unit area, and the shear rate, the difference in velocity between the two surfaces divided by their separation. However, it is often more convenient to measure a quantity known as the kinematic viscosity, which is the dynamic viscosity divided by the fluid density and is measured in mm2 s-1 or centiStokes (cSt).
Lubricants fall into two broad categories – monograde and multigrade – depending on whether their viscosity changes significantly with temperature or not. A more detailed classification system has been devised by the Society of Automotive Engineers (SAE) (see table). One common lubricant is described according to this scheme as an SAE-10W/30 multigrade. The first number (10W) refers to the dynamic viscosity measured at low temperatures, while the second (30) describes the kinematic viscosity at 100 oC. Lower numbers describe runnier lubricants – the viscosity of an SAE-5W/30 multigrade, for example, is five times lower than that of SAE-20W/50 at -20 °C. Roughly speaking, the energy lost due to friction varies with the square root of the viscosity: at -20 °C the friction losses of the low-viscosity oil will be approximately half those of the thicker oil, allowing the engine to start more easily.
The viscosity grade of a multigrade oil is different at high and low temperatures due to additives known as viscosity modifiers. For example, SAE-10W/30 has a similar viscosity to the monograde lubricant SAE-30 at 100 °C. At lower temperatures, however, SAE-10W/30 is much thinner than the monograde oil. This means that the multigrade oil provides protection at high temperatures and is runny enough at low temperatures to enable engines to start easily in most European countries. In contrast, the thick monograde oil would simply be unsuitable in winter.
| Viscosity grade | Kinematic viscosity at 40 °C (cSt) | Kinematic viscosity at 100 °C (cSt) | Dynamic viscosity at -15 °C (mPa s) |
| SAE-30 | 91.3 | 10.8 | 3950 |
| SAE-20W/50 | 144.8 | 17.8 | 5870 |
| SAE-15W/40 | 114.3 | 14.9 | 2940 |
| SAE-10W/30 | 72.3 | 10.8 | 1900 |
| SAE-5W/30 | 57.4 | 9.9 | 1090 |
| SAE-0W/20 | 44.4 | 8.3 | 690 |
The typical viscosities of common lubricants as graded according to the Society of Automotive Engineers’ J300 classification. A multigrade oil described as SAE-15W/40, for example, is a grade 15 at low temperatures and a grade 40 at high temperatures. For an oil to be a grade 15, the dynamic viscosity at -15 °C has to be less than 3500 mPa s, while the kinematic viscosity of a grade 40 oil must be between 12.5 and 16.3 cSt at 100 °C.
Well-oiled components
In a typical gasoline internal-combustion engine, fuel enters the combustion chamber when an inlet valve opens. This valve then closes and the piston moves upwards, compressing the fuel-air mixture. When the piston reaches its highest position, the spark plug is activated and combustion occurs, pushing the piston back downwards. The translational motion of the piston is then converted into rotational motion via the connecting rod and the crankshaft bearings.
For an engine to work effectively, the contacts between the cams (which push the valves controlling the inlet and outlet to the combustion chamber) and the tappets (which operate the valves) must be adequately lubricated so that the components do not wear excessively. A complete oil film must also be formed between the piston rings and the piston liner to prevent wear, to seal the combustion-chamber gases from the rest of the engine, and to minimize friction losses. Finally, a thick film of oil must cover the engine bearings so that the metal surfaces in the bearing do not come into contact.
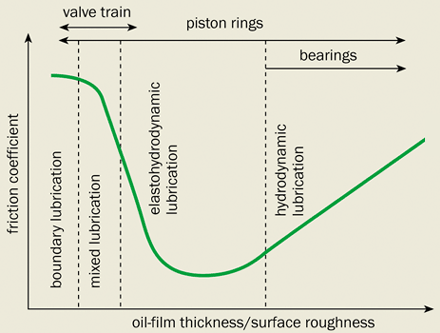
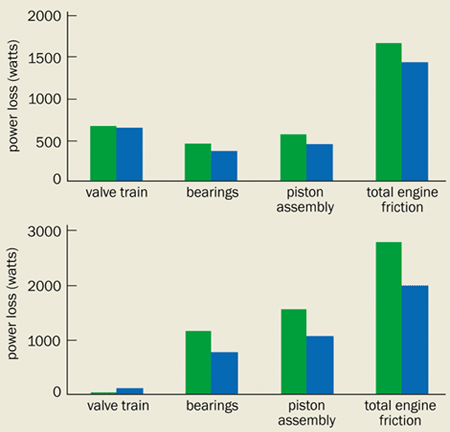
A typical engine oil is carefully formulated to protect the valve train, the bearings and the piston assembly, despite the different lubrication requirements of these components. In fact, the thickness of the oil determines its coefficient of friction and defines four distinct regions of lubrication (figure 1).
Engine bearings and the piston mostly operate in the “hydrodynamic lubrication” region, where a thick film separates the moving metal surfaces so that there is no chance of them coming into contact. When the pistons are momentarily stationary, however, the layer of oil covering them can be similar in thickness to the surface roughness of the components. In this “mixed lubrication” region, the metal surfaces intermittently come into direct contact. If the thickness of the oil film is much smaller than the surface roughness then the metal surfaces rub together repeatedly – this is known as “boundary lubrication”. Contact between the cams and the tappets in the valve train span the mixed and boundary regions.
The final type of lubrication – elastohydrodynamic lubrication – occurs under high loads and is commonly encountered with hydrocarbon-based oils. Here the pressure developed in the lubricant is sufficiently high to elastically deform the metal surfaces either side of the oil film. This happens because the viscosity of these fluids increases significantly as the pressure rises. The valves and the piston rings occasionally operate in this region.
Lubricants are also used in other important components in a vehicle, including the gearbox. This is a challenging environment where pressures routinely exceed 109 Pa and the gears operate in the elastohydrodynamic-lubrication regime. Moreover, in many cars the gear lubricant is filled once and then never replaced during the vehicle’s lifetime. Even so, researchers at Torotrak in Leyland, UK, are currently developing novel transmission systems that are effectively gearboxes with an infinite range of gear ratios. These continuously variable transmissions are designed to ultimately improve fuel efficiency and they require lubricants that have special properties, such as very high friction coefficients, which our group at Shell’s Cheshire Innovation Park is developing in collaboration with Torotrak.
Greases are also commonly used to lubricate the constant-velocity joints that connect the axles to the drive wheel while allowing the suspension to move up and down. These joints are critical components in many current models of four-wheel-drive and sports-utility vehicles and they therefore require high-performance greases.
Measurements smooth the way
Most engine manufacturers design their components to operate with oils and greases within a certain viscosity range. It is therefore important to be able to measure the properties of lubricants accurately.
The kinematic viscosity of oil is usually determined under low shear rates, simply by measuring the time the meniscus takes to flow vertically downwards between two marks on a capillary tube. Different diameter capillary tubes are used for thinner or thicker oils.
Meanwhile, the dynamic viscosity is usually measured under the high shear conditions – and sometimes the high temperatures – typically found in bearings and other critical contacts in engines. These measurements are carried out by instruments in which a thin film of oil is trapped between two surfaces moving relative to each other. For example, in a “rotating cylinder viscometer”, the dynamic viscosity is estimated from the shear torque produced on a stationary inner cylinder by a revolving outer one. Another instrument that is used for measuring very small quantities of lubricant, in particular, comprises a conical surface rotating against a flat plate coated in oil.
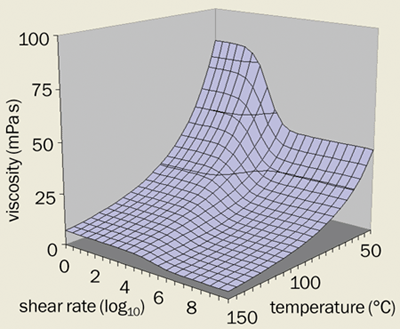
These measurements can also tell us something about how the viscosity varies with shear rate. Our group at Shell and others, including Jagadish Sorab at Ford, have fitted such data to realistic equations that describe how the viscosity varies with both temperature and shear rate (figure 2). We have found, for example, that the viscosity of a typical engine lubricant decreases at high shear rates. The reason is that the large polymer molecules used as viscosity modifiers line up in the direction of the shear force at high shear rates. This alignment reduces the “thickening” effect that these polymers have when they are randomly aligned.
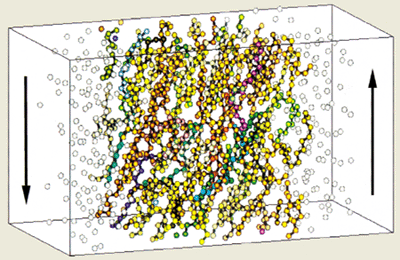
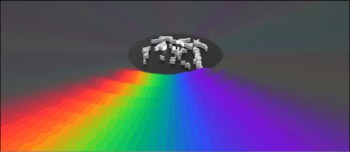
Indeed, non-equilibrium molecular-dynamics simulations of model lubricants carried out by physicists at Shell in the early 1990s clearly show how the molecules line up when a high shear rate is applied to the fluid (figure 3). The effect, however, is temporary – the viscosity returns to its previous value when the shear rate is reduced.
Crucially for automotive applications, these types of experiments have also revealed how the viscosity of a lubricant varies with pressure. For example, the viscosity of a typical lubricant at 500 MPa can be between 10 000 and 100 000 times higher than that at atmospheric pressure. Roughly speaking, the viscosity increases exponentially as the pressure increases. This exponential variation is known as Barus’s law and is valid at pressures up to a few hundred megapascals. At very high pressures (2-4 GPa), however, this simple relationship tends to overestimate the increase in viscosity. Instead the lubricant becomes glass-like and behaves more like a solid than a liquid, deforming the metal surfaces on either side of the lubricant elastically – this is the elastohydrodynamic-lubrication regime.
Elastic effects
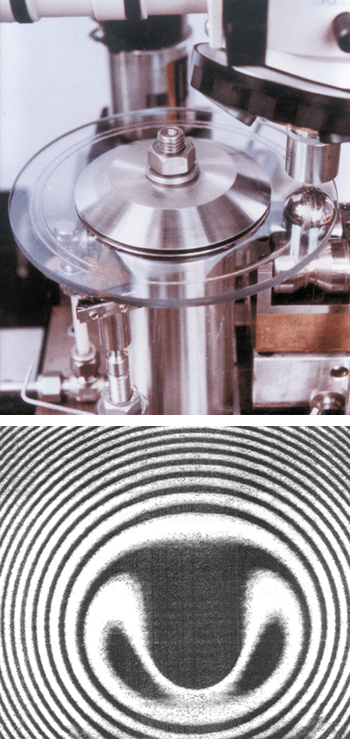
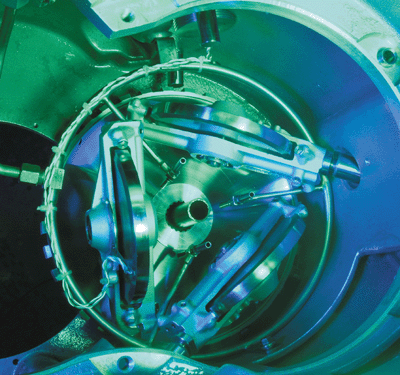
Other optical and mechanical techniques have been developed to investigate the behaviour of oils and greases in the elastohydrodynamic region. One of the most common instruments used for this purpose is the so-called ball-on-plate rheometer, which was first used extensively by Hugh Spikes’ group at Imperial College in London in the 1980s. Essentially the instrument consists of a steel ball that is pressed against a transparent rotating disk made of glass or sapphire. As the disk rotates, the thickness of the oil film between the two components is measured by shining light through the transparent disk and monitoring the resulting interference fringes (figure 4, top).
When the ball is pressed lightly against the disk, the surface remains unaltered and a series of concentric circles is observed. However, if the ball is pressed hard against the disk, then the plate deforms elastically, as shown by the characteristic horseshoe-shaped interference pattern (figure 4, bottom). This shape indicates that the thickness of the oil film at the centre point of the contact is approximately constant, while the increase in the number of fringes outside the central region demonstrates that the film is thicker at the edges. In this elastohydrodynamic region, the flat central region behaves in a similar way to a ball of Plasticine pressed against a flat surface, with the difference that the surfaces return to their original shape when the pressure is removed.
Elastohydrodynamic lubrication occurs in gears, valve trains and in the “rolling-element bearings” found in wheel hubs, which have to cope with large radial and thrust loads with minimum friction. The ball-on-plate rheometer is therefore ideal for evaluating the performance of lubricants under the realistic conditions found in many machine components.
Other methods for measuring the viscosity of lubricants under high pressures include the “falling ball” experiments pioneered by Bo Jacobson, now at Lund University in Sweden, and co-workers in 1985. In these experiments, a steel ball is dropped either vertically, or more commonly at an angle, onto a plate smeared with a drop of oil or grease. Pressures of up to 7.5 GPa can be created in the lubricant film in this way. And the coefficient of friction can be determined from either the motion of the ball after contact, or from force transducers on the surface. Different lubricants have different friction coefficients because of the way that the viscosity varies with temperature, shear rate and pressure.
Finally, it is worth mentioning that many lubricants also exhibit some elastic effects – in other words their behaviour cannot be fully explained just by assuming that they are purely viscous fluids. Grease, for example, is viscoelastic: under certain conditions it behaves as a viscous fluid – for instance when it flows freely in a pipe under an applied pressure; on other occasions, it behaves like a solid, for example prior to flowing. Most other commercially available lubricants also exhibit viscoelastic behaviour, albeit to a lesser extent.
Rheometers can also be used to measure viscoelastic properties as well as viscosities. However, little is known about how these attributes vary with temperature, pressure and shear rate because these measurements are difficult to make for commercial lubricants that have weak viscoelasticity. However, several researchers – including Brian Williamson at Shell and Ken Walters at the University of Wales in Aberystwyth – currently speculate that viscoelastic lubricants form thicker oil films in engine bearings under extreme conditions than less elastic fluids.
Grease-lightning simulations
With the increased computer capacity that has become available over the last decade, it is now possible to accurately model the performance of lubricants in engines, gearboxes and other components. For example, if we know the minimum thickness of the oil film, we can predict the durability of the component and the power loss due to friction. This capability can help researchers who are designing new lubricants to predict how a machine’s performance is related to lubricant viscosity, and how it will be affected by temperature, shear rate and pressure.
The theory of elastohydrodynamic lubrication was first developed by Duncan Dowson at Leeds University, and others, in the 1950s, when computer simulations were not possible. In elastohydrodynamic lubrication both the fluid equations and the elastic deformation of the surfaces have to be modelled, and these complicated simulations can now be performed on modern computers relatively quickly. Meanwhile, hydrodynamic lubrication in plain bearings and piston rings can be analysed in seconds. Modelling mixed and boundary lubrication is more difficult since we require a detailed understanding of the roughness of the surfaces as well as of the lubricant properties. In general, the output of the models is the minimum thickness of the oil film and the losses due to friction.
Many groups have accurately estimated the minimum oil-film thickness in an elastohydrodynamic contact. However, estimating the friction coefficient has proved much more problematic due to the large variation of viscosity with pressure, which is not always accurately known. Recently, Laurence Scales at Shell has shown that it is worth investing the effort to find accurate relationships between viscosity, temperature, pressure and shear rate because they allow both the minimum oil-film thickness and the friction coefficient to be estimated. To do this, however, requires at least eight parameters to characterize the lubricant.
But by combining models for the plain bearings, the piston assembly and the valve train, researchers have found that they can model the lubrication conditions in a complete internal combustion engine. Since the bearings and piston rings are lubricated predominantly in the hydrodynamic regime, a lubricant with a lower viscosity should lead to a thinner oil film and thus lower friction. However, the valve train operates in the mixed-boundary lubrication regime, which means that lower friction can only be obtained with thicker lubricants. Engine-friction models enable us to study the trade-off between viscosity and friction, and therefore select the optimum lubricant to improve a vehicle’s fuel economy (figure 5).
Future challenges
There are many challenges in developing the lubricants of the future. Cars are becoming more powerful, drivers would like to change the engine oil less frequently, and manufacturers want to reduce the losses due to friction even further. In order to develop lubricants that satisfy these demands, physicists and engineers have to understand the performance of the lubricant in more detail.
First, we need to fully understand the role that the elastic properties of lubricants play under extreme conditions. To do this we will need to measure the viscoelastic properties of lubricants at different temperatures, pressures and shear rates, and develop a suitable model. Our current models are based on the Reynolds’ equation, which assumes that the oil film is of the order of a few microns thick and that the components are a few millimetres across. A more serious shortcoming of the Reynolds’ equation is that it assumes that elastic effects are not important.
The second major challenge is to incorporate chemistry into physical models. After all, lubricants change chemically during their time in an engine. Simply speaking, a fresh lubricant is like a pure hydrocarbon. Over time, however, it oxidizes and chemically degrades to form alcohols, ketones, aldehydes, acids and esters. These chemical changes can lead to an increase in viscosity. The present author is currently developing a chemical model that also simulates the reactions in an engine, in collaboration with Martin Priest at Leeds University and John Lindsey-Smith at York University, both in the UK. The aim is to determine the chemical composition of the lubricant in the sump at any instant and, in principle, the increase in viscosity, which would then feed into the physical models discussed earlier. However, this still leaves the challenge of understanding the physics and chemistry in the boundary-lubrication region.
Safeguarding the environment
Physicists are playing an increasing role in addressing the environmental problems of pollution and global warming, as well as in understanding natural climate phenomena such as El Niño. Ever-more-sophisticated experiments are revealing that lubricants are a rich source of physics, and physicists are quick to use these findings to design environmentally friendly lubricants that will help to reduce our impact on the planet. The progress that is being made in lubricant research today will undoubtedly play a part in safeguarding the natural environment for many generations to come.