When one bad apple rots the bunch, it’s ethylene’s fault. Not only does this “universal plant hormone” trigger germination, flowering, ripening and rotting in seeds, flowers, fruit and vegetables, it’s also released during these processes, ensuring that a small problem quickly escalates. Ethylene is social media for plants, allowing them to communicate and synchronize – and it’s something the food and flowers industries would love to detect early, so they can forecast which products to sell first, and how to spot rot before it spreads.
The problem? “There really isn’t a good industrial sensor out there,” says Timothy Swager, a materials chemist at the Massachusetts Institute of Technology (MIT) in the US. Now, however, Swager, Darryl Fong and colleagues at MIT and the Nanotechnology National Laboratory for Agriculture in Brazil have combined the sensitivity of carbon nanotubes (CNTs) with a highly selective catalyst to produce a sensor that can detect ethylene at concentrations of as little as 15 parts per billion.
CNT sensing
Previous approaches to ethylene sensing have typically either been based on photoacoustic spectroscopy (which detects sounds produced in response to light) or on gas chromatography (which separates chemicals based on their different retention times in solvents). Neither technique is easy or simple enough for a grocer or florist to incorporate it into their work. Instead, Swager and colleagues looked at the reactions ethylene readily undergoes and focused on finding ways of detecting when such a reaction had taken place.
This is where the CNTs come in. Single-walled CNTs have several attributes that make them well-suited for sensing processes that involve the transfer of electrons – the basis of any chemical reaction. The CNTs Fong and Swager and their colleagues worked with are p-type semiconductors, so n-type dopants – anything that donates electrons to the CNT – will diminish their conductivity. The CNTs’ curved graphene surface also makes their electronic properties incredibly sensitive to dopants in their environment.
This may sound ideal for a sensor, but you can have too much of a good thing. “There’s a graveyard of CNT sensors with low specificity,” Swager tells Physics World. Because the CNTs are so sensitive to everything in the environment, he explains, all you detect is white noise. “You need a way of boosting the signal above the noise,” he adds.
Wacker reaction chemistry
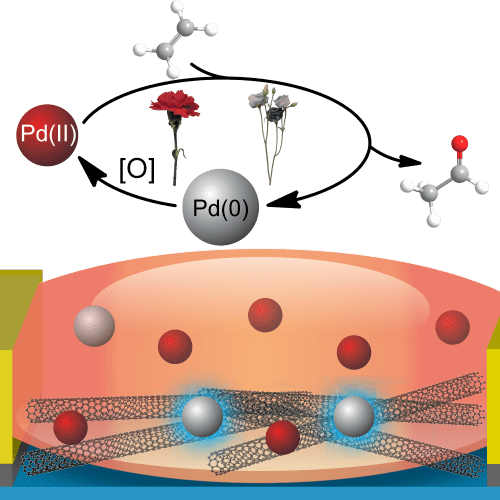
Building on their expertise in analytic and synthetic chemistry as well as CNT technology, Swager and colleagues identified the so-called Wacker reaction as having both the specificity and sensitivity they needed. In this reaction, which was developed as a synthetic process in the 1950s, ethylene – a hydrocarbon containing two double-bonded carbon atoms – oxidizes into acetaldehyde. This chemical is perhaps best known as the primary cause of hangover-related headaches, although the researchers note that the test they developed does not produce it in high concentrations.
The Wacker reaction is not the only reaction ethylene undergoes, and it was not the first port of call for Swager and his group. First, they tried to mimic the plants themselves, which use copper ions to promote ethylene activity. However, trying to make a sensor out of copper proved almost as headache-inducing as acetaldehyde. Plants have a natural ability to keep copper in its Cu(I) oxidation state, which has one fewer electron than the material would have as a neutral atom. Ethylene readily binds to copper in this oxidation state, triggering a cascade of plant activity and ultimately the up-regulation of certain genes. However, in a synthetic device, copper tends to exist in the Cu(II) oxidation state, and preventing Cu(I) from oxidizing to Cu(II) proved too difficult for this approach to have commercial potential.
In the Wacker reaction, in contrast, ethylene oxidation is catalysed by palladium in the Pd(II) state. This is far more stable than Cu(I). Although palladium can catalyse other reactions as well, the palladium in the Wacker reaction is in an organic complex optimized to catalyse ethylene oxidation. The reaction also relies on a nitrite source, which contributes to the organic complex. The resulting ethylene reaction surrenders electrons that reduce Pd(II) to the n-dopant Pd(0), which the highly sensitive CNTs should announce with a dip in conductivity from the excess electrons.
Budding commercial potential
The researchers tested their proposed mechanism by replacing the CNTs with ZnO nanofibers, which are n-type semiconductors. This test showed that, as expected, the material’s conductivity increased in response to the Pd(0) produced in the presence of ethylene and the Wacker reaction Pd(II) catalyst. Next, they optimized their CNT-based sensor and used it to detect ethylene production from carnations (which, in their experiment, all burst into bloom on the same day) and purple lisianthus (which bloomed over the course of a week). They found that the ethylene they detected reflected these different blooming times.

Carbon nanotubes make molybdenum disulfide more active
The process is now licensed by a company that Swager co-founded, but some hurdles remain before the sensor is available commercially. “We could industrialize these sensors in a matter of months, but how long before it is commercialized is something the business world will control,” Swager says.
The researchers describe their work in ACS Central Science.



