Home »
Materials » Catalysis and chemistry » Cheap electrocatalysts convert CO2 to CO using solar cells
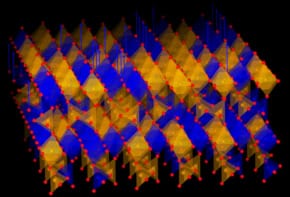
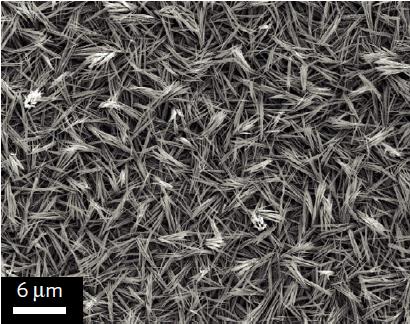
Cheap electrocatalysts convert CO2 to CO using solar cells
04 Jul 2017 Pamela Carrillo
Pamela Carrillo
is a former PhD student contributor to Physics World. Pamela studied physical chemistry at Stony Brook University, US. Find out more about our student contributor networks