An artificial intelligence (AI) system that can identify diabetic retinopathy (DR) without physician assistance, including the most serious form that puts patients at risk of blindness, has outperformed expectations in a clinical trial. The commercial system successfully detected the presence and severity of the disease in 97% of eyes analysed. Deployment of such AI systems in primary care facilities for use by non-specialists could significantly increase access to eye exams that include DR evaluation, aiding in the diagnosis and treatment of the disease.
DR is the most common cause of preventable vision loss and blindness in adults aged 20 to 65. More than one third of all diabetic individuals are expected to develop the disease, according to the International Diabetes Federation (IDF). The IDF also estimates that almost half of the approximately 537 million people in 2021 with diabetes are undiagnosed, and therefore may not know that they are at risk of developing DR.
DR occurs as a result of damage to blood vessels and neurons in the retina caused by high blood sugar. Fluctuations in blood sugar cause constriction of the retinal arteries, reducing blood flow and leading to dysfunction of neurons located in the inner retina. This dysfunction tends to spread to neurons of the outer retina and the blood–retinal barrier that protects the retina from toxins. When the barrier begins to leak fluid, damage to vital neurons occur. The early – and most treatable – stages of DR are often asymptomatic, or present as changes in vision that may occur and disappear and be incorrectly attributed to ageing. The risk of developing DR increases the longer a person has diabetes.
In addition, around 80% of all diabetic individuals live in low- and middle-income countries, where access to professional eye examinations and specialists with the expertise to identify DR may be limited or unaffordable. According to the Diabetic Retinopathy Barometer Report Global Findings, 21% of patients with diabetes worldwide have never undergone DR screening.
Assessing AI performance
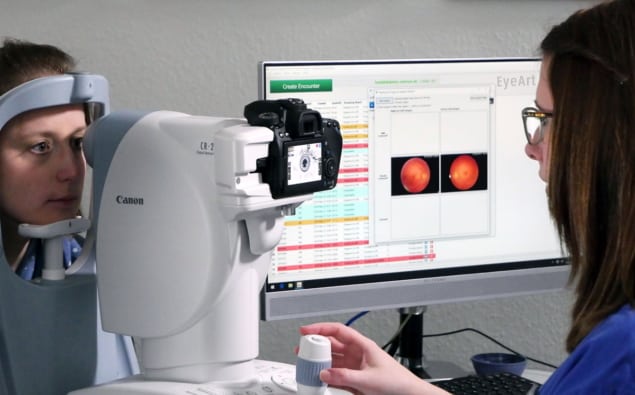
Researchers at the Lundquist Institute for Biomedical Innovation, in collaboration with 14 other US centres, conducted a clinical trial to evaluate the safety and ability of the EyeArt Automated DR Detection System to autonomously detect more-than-mild DR and vision-threatening DR, using dilated (in which the pupils are widened) and undilated eye imaging regimes.
The trial included 942 diabetic patients (893 of whom completed the study protocol) who underwent eye exams at six primary care centres, six general ophthalmology centres and three retina speciality centres located throughout the US. The participating facilities represent a wide range of geographic locations and an urban/rural mix. The study aimed to incorporate all ethnicities, even though the AI system’s algorithm neutralizes for colour and light differences to minimize racial differences in retinal hues.
The patients initially had eye exams comprising two-field (disc-centred and macula-centred) retinal colour fundus photography (CFP) of each undilated eye. Following dilation, patients then underwent four-wide-field stereoscopic CFP imaging, in accordance with the Wisconsin Reading Center (FPRC) reference standard. Two independent readers masked to the AI results examined the images using standardized procedures to establish the reference standards and categorize images as negative, mild-to-moderate DR or vision-threatening DR. The researchers then compared the readers’ findings with the AI system grading.
Writing in JAMA Network Open, lead author and principal investigator Eli Ipp reports that use of the EyeArt system in both primary care and eye care centres compared favourably with the reference standard in detecting both categories of DR.
With the undilated imaging protocol, the AI system exceeded predetermined end points for both sensitivity (greater than 90%) and specificity (greater than 82.5%) of DR detection. The sensitivity of detecting more-than-mild DR in undilated eyes was 96%, with a specificity of 88%. For vision-threatening DR, the system exhibited a sensitivity of 97% and specificity of 90% in undilated eyes. The AI system was able to grade 97.4% of the eyes, with 87.6% not requiring dilation.
“To our knowledge, this study is the first to investigate the ability of an AI system to identify vision-threatening DR,” explains Ipp. “The system was easy to use at primary care centres, and with standardized training, reliable disease-detection results can be obtained by staff who have no prior retinal imaging experience.”
He points out that the system allows eye exams to be more easily performed, because the majority of patients in the study did not need to have their eyes dilated for the AI system to evaluate the colour fundus photographs accurately.
“By simplifying and improving the efficiency of diabetes retinal screening, this technology has the potential to contribute to saving vision for millions of people around the world,” Ipp concludes. “Our clinical study reinforces the capability of this AI system to diagnose early as well as advanced cases of DR, which should reassure healthcare professionals considering its use.”
- The EyeArt system evaluated in this study has received 510(k) clearance from the US Food and Drug Administration, a Health Canada license, validation from the UK National Health Service, and CE Marking as a class 2 medical device by the European Union. It is currently being used globally, including at healthcare facilities in economically challenged countries.