Dispersible electrodes based on gold-coated magnetic nanoparticles modified with DNA can detect microRNA in unprocessed blood samples at extremely low concentrations and over a broad range – a first for sensors of this kind. The devices, which have been tested on mice, can produce results in just 30 minutes and might be used to make a finger-prick test for early-stage cancer diagnosis.
“There are many microRNAs (short ribose nucleic acid sequences between 19 and 25 bases long) that are post-transcriptional gene expression regulators – that is, they can turn genes on and off,” explains John Justin Gooding of the University of New South Wales in Australia, who led this research effort. “The levels of these miRNAs are indicative of a range of pathologies, including cancers. If we could detect these RNAs in blood, where they circulate, we could make a finger-prick test as an early cancer diagnostic.”
Detection levels as low as 10 attomoles
“The problem is that the RNAs are found at very low concentrations of 10 femtomoles (fM) to 1 picomole (pM), so this is no easy task. Our new technique can detect levels as low as 10 attomoles (aM) and above 1 nanomoles (nM), so covering this entire range. What is more, it produces a result in just 30 minutes.”
Gooding and colleagues developed gold-coated magnetic nanoparticles (Au@MNPs) modified with DNA that is complementary to the miRNA they want to detect. “We call these magnetic nanoparticles ‘dispersible’ electrodes because they diffuse throughout the sample to capture the miRNA,” says Gooding. “When we then apply a magnetic field, these tiny electrodes reassemble to form a bigger electrode.
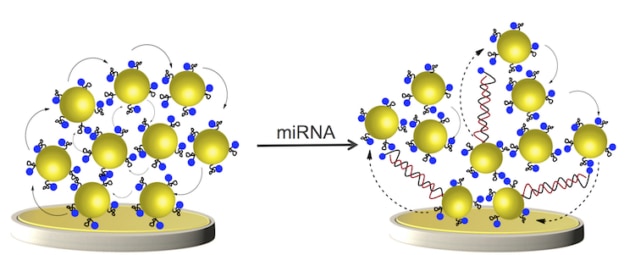
“When miRNA is bound to the DNA of the nanoparticles, the electrochemical current through the macro-electrode changes. The electrode measures this change and produces a signal,” he explains. “Since the nano-electrodes disperse throughout a sample, they capture nearly all the miRNA in it and the more they capture, the bigger signal. This is why our device is so sensitive.”
Fast response time
The sensor also has a fast response time since it makes use of an applied magnetic field to “bring back” all the captured miRNAs to the macro-electrode, he adds. “It can be likened to a hunter-gatherer that is on a motorcycle rather on foot. When sent out to find ‘food’, it covers more territory, so collects more food and brings it back faster.”
The technique is better than the current gold standard to profile miRNA, the real-time polymerase chain reaction (qRT-PCR), which does not work on samples of whole blood (it requires isolated and purified RNA). Although highly reliable, qRT-PCR is also labour-intensive and time consuming.”
“Our sensor is the first to be able to detect concentrations of miRNA from 10 aM to 1 nM in unprocessed blood samples,” Gooding tells Physics World. “We found that it can also distinguish small variations in miRNA concentrations in blood samples taken from mice with growing tumours.
“We believe that our work is an important advance for developing liquid biopsies for early cancer detection, that is before symptoms of the disease actually appear, and to monitor how well, or not, a treatment is working,” he adds.
The researchers, reporting their work in Nature Nanotechnology 10.1038/s41565-018-0232-x, will now be trying to multiplex the technology so that they can simultaneously measure different types of miRNAs.