Cell therapy is a promising approach for the treatment of many degenerative diseases. For instance, chondrocytes (cartilage cells) are currently being tested in clinical trials to treat intervertebral disc degeneration, cartilage disease and osteoarthritis. However, the localized delivery of cells still poses some important challenges.
For example, when injecting the cells, most of them can be lost from the affected zone through diffusion to other parts of the body. Meanwhile, the large needles used can cause further damage in the degenerated intervertebral disc. Another limitation is the survival of the cells, which is usually quite low and limits the efficiency of the treatment.
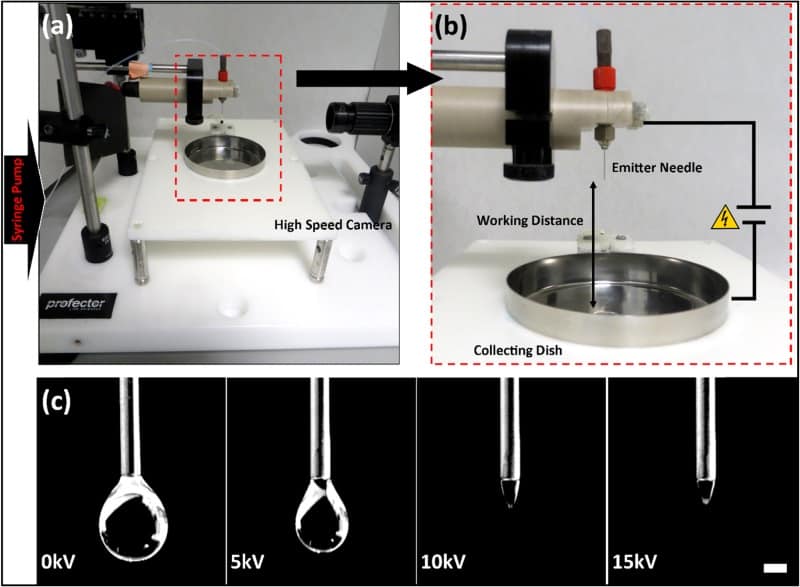
Given these limitations, Conor Buckley and his team from Trinity College Dublin have recently characterized an electrodynamic spray system to encapsulate chondrocytes for delivery to degenerated intervertebral discs (Biofabrication 10 035011). To do this, they optimized the conditions to “trap” and deliver the cells and tested their effects on the viability of the chondrocytes.

How does electrodynamic spraying work?
The researchers used an electrodynamic spray microencapsulation system to “trap” cells in protective microdroplets of alginate, a natural polymer. This technique is based on the application of an electric voltage to a needle while pumping through a polymer solution containing cells. The electric potential in the needle overcomes the surface tension forces of the polymer solution and creates a cone at the end of the needle, called a Taylor cone. From this cone, a jet of microdroplets is ejected and collected, containing cells for further application.
However, multiple variables can influence the formation and properties of such microcapsules. Therefore, the team analysed various processing conditions – such as needle gauge size, electrical charge and pumping flow – to understand how they influence the microcapsules.
Playing with flow, charge and needles
The first stage was to assess how the combinations of the different conditions affected the alginate microcapsules. The researchers found that increasing the voltage and decreasing the needle size decreased the diameter of the particles but resulted in some variability in their size.
Small particle size is a desirable characteristic since it allows use of a smaller needle when injecting the cell-loaded microcapsules into the intervertebral disc, thus decreasing damage to the patient’s intervertebral disc. Moreover, although size homogeneity in the particles could provide positive features such as a more controlled diffusion, the observed slight variation also permits a higher packing density and therefore delivery of denser material.
Increasing the concentration of the polymer increased the particle size, whereas increasing the flow produced more ellipsoidal rather than spherical particles, with no effect on the size. However, increasing the polymer concentration also decreased cell viability, which the authors attributed to the shear forces caused by higher concentrations.

Based on these results, the researchers chose an operating set up of 10 kV, a 30 gauge (G) needle, 1% alginate and a flow rate of 0.1 ml/min. This parameter set enabled microcapsule injection with a 25G needle, without affecting cell viability, making it suitable for cell delivery.
What about the cells?
Once the set up was optimized, the team next determined how many cells the microcapsules can carry and how this affects their behaviour. They tested three different initial cell densities: 5 million, 10 million and 20 million cells per ml.
They observed that higher densities decreased cell viability, which can be explained by cell-to-cell signalling and/or by the depravation of nutrients due to the high number of cells consuming them. In fact, the low availability of nutrients is a characteristic of the intervertebral disc environment.
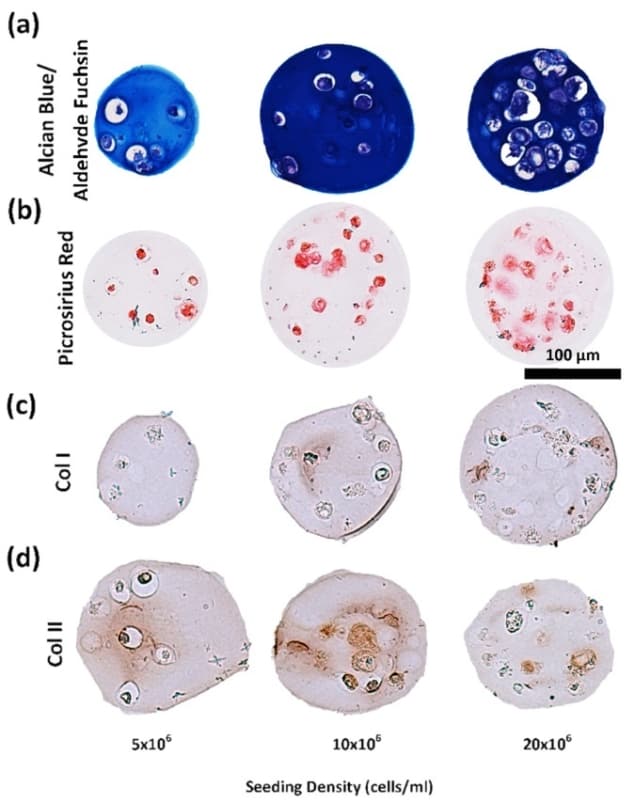
The researchers also tested whether the cells were producing the main components of the intervertebral disc microenvironment: collagen and glycosaminoglycans (sugar polymers highly present in intervertebral disc and cartilage). They found that chondrocytes in the alginate electrosprayed microcapsules did indeed produce such components, at higher amounts from the high cell density samples, but more efficiently (more collagen/glycosaminoglycan per single cell) at low cell densities.
Moreover, the cells produced these components in a very similar ratio to that found in an intervertebral disc. The authors concluded that the 10 million cell per mL density presented the best balance between viability and intervertebral disc component production.
A minimally invasive cell-delivery system
This study adds important knowledge to the electrodynamic spray microencapsulation technique and may help in the development of further systems employing this powerful approach. The researchers successfully developed a chondrocyte-encapsulation system that preserves the viability of the cells and promotes the deposition of key components of the intervertebral disc microenvironment. In addition, such a system can be delivered through minimally invasive systems, representing a high potential for (though not limited to) intervertebral disc cell therapy.



