
Targeted radionuclide therapy (TRT) is an emerging cancer treatment in which radiopharmaceuticals travel through the bloodstream and selectively bind to cancer cells. Once within the tumour, the radioactive nuclides emit alpha or beta particles that deposit their energy in a localized region and destroy surrounding cancer cells.
Current TRT approaches rely on the presence of unique receptors on the surface of cancer cells, to which the radiopharmaceutical can bind. This targeting means that the drug is only delivered to the cancer cells, sparing healthy tissue and organs. Tumour heterogeneity and cell mutation, however, can alter the receptor profile, making it difficult to devise effective targeting strategies.
To address this obstacle, researchers at the University of Cincinnati are developing a novel TRT delivery approach that could target and ablate multiple tumour types, irrespective of their receptor phenotype. In a proof-of-concept study, reported in Advanced Healthcare Materials, they show how tumour-colonizing bacteria can attract a bacteria-specific radiopharmaceutical into cancer cells, even those with no receptors to target.
Senior author Nalinikanth Kotagiri and colleagues genetically engineered the probiotic Escherichia coli Nissle (EcN) to overexpress a metal uptake receptor on its surface. The engineered bacteria, which can be delivered into solid tumours, is then used to attract a bacteria-specific radiopharmaceutical comprising yersiniabactin (YbT, a siderophore molecule that binds to metals) labelled with the therapeutic radioisotope 67Cu.
“As long as these engineered bacteria are inside a tumour, these targeted agents, specific to the bacteria, will transport the radioactive metal,” says Kotagiri in a press statement. “They won’t care if there is a cancer cell that is expressing a receptor or not. All they care is that they have identified something that they can recognize, accumulate and retain in.”
What’s more, replacing 67Cu with 64Cu, which can be visualized via positron emission tomography (PET), enables tracking of the bacteria location inside the tumour. “We can make this switch between copper-64 and copper-67 seamlessly to image the tumour, and then once we have imaged, we can introduce another molecule again to do therapy,” Kotagiri explains.
Bacterial accumulation
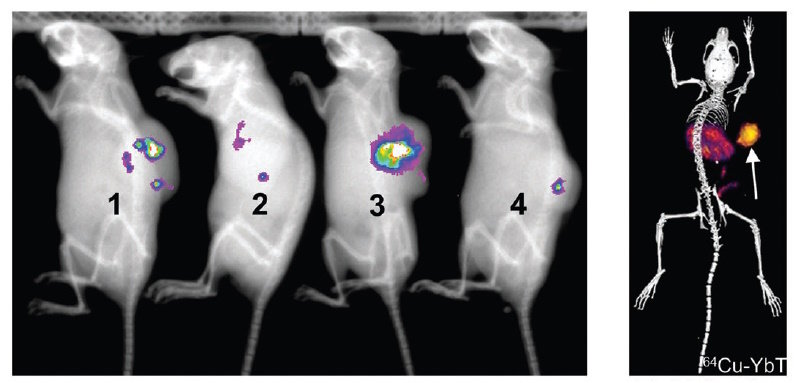
The researchers first used PET/CT to examine the accumulation of the engineered EcN in vivo. They injected the bacteria directly into colon cancer tumours in mice, and then administered 64Cu-YbT. Bioluminescence imaging confirmed bacterial localization in the tumour, while PET/CT revealed significantly higher signals in tumours containing engineered EcN versus a bacteria strain that does not express the metal uptake receptor. They note that the 64Cu-YbT probe was cleared primarily by the liver and kidneys with minimal accumulation in other major organs.
Examining harvested organs revealed significant bacterial presence exclusively in the tumours at one and seven days after administration. Bacterial levels in all major organs were below the limit of detection. In a separate group of mice, two days after bacteria administration, the researchers observed approximately ten times more bacteria in the tumours than the amount injected 48 h earlier.
On day 18, when the tumour was fairly large, both bioluminescence imaging and PET/CT confirmed that the engineered bacteria not only maintained their population inside solid tumours, but also achieved sustained growth, consistent with the growth of the tumour itself.
Testing the tumour therapy
Next, the researchers switched the 64Cu for the high-energy-beta-emitting isotope 67Cu. In mice with breast or colon cancer, they injected saline or engineered EcN directly into the tumour, followed by administration of 67Cu-YbT one and four days later. For both tumour models, mice treated with the combination of bacteria plus 67Cu-YbT had reduced tumour growth and survived significantly longer than those receiving either saline, bacteria only or 67Cu-YbT only.
In the colon cancer model, the combination treatment extended the median survival of mice with highly aggressive tumours from eight days (after initiation of treatment) in the control groups to 13 days. In mice with breast tumours, the median survival improved from 11 days in the control groups to 18 days in the combination group.
Targeted treatment guides radiation directly to pancreatic tumours
The team also evaluated the immune cell profile of the cancer microenvironment seven days after treatment. Following systemic administration of 67Cu-YbT, infiltration of cytotoxic CD8+ T cells increased significantly. Combined with a reduction in immunosuppressive regulatory T cells (Treg) upon delivery of bacteria, this led to a promising anti-tumour environment, with the CD8+:Treg ratio significantly higher following combination therapy than in the other three groups combined.
Kotagiri tells Physics World that the researchers now plan to express human receptors on the bacterial surface, enabling current FDA-approved targeted probes to be used and providing a quicker pathway towards potential clinical translation.
“For example, 177Lu-labelled DOTATATE and SARTATE are already approved agents that are used to target neuroendocrine tumours expressing somatostatin receptor,” he explains. “What about those patients that do not express this receptor? Can we use this technology to express the receptor on bacteria, and many other receptors, in a plug-and-play manner to accommodate the wide range of radiopharmaceuticals already approved?”




