Pancreatic cancer remains a leading cause of cancer‑related death worldwide. Patients are usually treated with chemotherapy or radiation therapy, but these are not always effective and can have toxic side-effects due to the treatments also impacting healthy cells.
A research collaboration between Osaka University and Heidelberg University Hospital is exploring an alternative approach: targeted radionuclide therapy, in which a radioactive molecule travels through the bloodstream to the tumour to deliver radiation directly to cancer tissue (J. Nucl. Med. 10.2967/jnumed.119.233122).
“With traditional anti-cancer therapies, there’s a trade-off between efficacy against cancer cells and off‑target effects in non‑cancerous cells,” explains lead author Tadashi Watabe from Osaka University Graduate School of Medicine. “We’re focused on finding ways to re-balance this trade-off in radiotherapy, by increasing the dose of radiation delivered to cancer cells while keeping it localized to those cells as much as possible.”
The new treatment employs the alpha particle emitting radionuclide 225Ac. Alpha particles travel a short distance in tissue, thereby limiting their off-target effect. The researchers targeted the therapy at the fibroblast activation protein (FAP), which promotes tumour growth and progression and is found almost exclusively on stroma cells surrounding pancreatic tumours and various other cancer types. The low expression of FAP in normal tissue makes it an excellent target for this approach.
The researchers used the positron emitter 64Cu and 225Ac to label small-molecule FAP inhibitor (FAPI) probes. They employed 64Cu-FAPI-04 to evaluate tumour uptake in mice bearing human pancreatic cancer xenografts, and 225Ac-FAPI-04 to assess radionuclide therapy of the tumours.
PET scans of mice injected with 64Cu-FAPI-04 revealed mild uptake in tumours and relatively high uptake in the liver and intestine, with rapid clearance through the kidneys and slow washout from tumours. Immunohistochemical staining revealed abundant FAP expression in the stroma of tumour xenografts, while cellular uptake analysis demonstrated minimal accumulation in the tumour cells themselves.
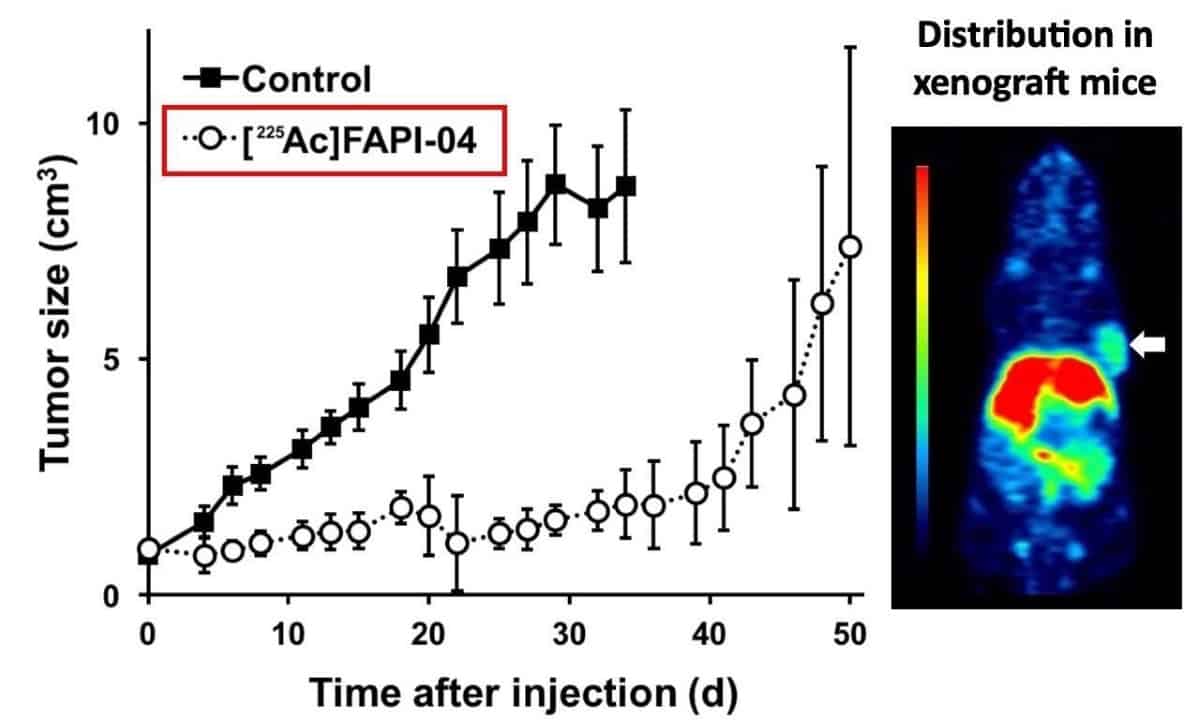
For the targeted therapy using an alpha emitter, the researchers injected 225Ac-FAPI-04 (at a dose of 34 kBq) into the tail veins of six tumour-bearing mice. They observed significant reduction in tumour growth in these mice compared with control mice. Importantly, the treatment did not significantly change the animals’ body weights, indicating that it likely had few toxic side effects.
“We are very encouraged by these initial results,” says Watabe. “We think the approach has enormous therapeutic potential, particularly for patients with pancreatic cancer who’ve exhausted their other treatment options. What’s especially exciting is that our method of targeting the stroma can in principle work against many other types of cancer. We think this could represent a new path forward in radiation therapy.”



