Quantum dots are responsible for the stunning, vibrant colours on modern TV screens, but they don’t always behave like they should. This creates headaches for device makers, who struggle to understand why some of these nanometre-scale semiconducting crystals shine much more dimly than others.
A team led by scientists at the SLAC National Accelerator Laboratory in the US has now used fast electrons to determine the cause of this bad behaviour. Their experiment – the first direct observation of its kind – probed the atomic structure of inefficient quantum dots in real time as they were (not) working, demonstrating conclusively that their inefficiency stems from energy-sapping distortions in the dots’ crystal lattice.
Quantum dots absorb light at one frequency and emit it at another. The ones in TV screens are very efficient at this, emitting almost as much light as they absorb. Their efficiency drops significantly, however, in devices such as ultraviolet photodetectors that use very energetic and bright light. Worse, when scientists try to make quantum dots brighter, the dots react by darkening instead.
Understanding inefficiency
Aaron Lindenberg, a materials-science researcher at Stanford University and a co-author on the study, explains that when quantum dots absorb photons, not all of the photon energy goes into the re-emitted light. Some of it gets wasted on processes that keep the quantum dot dark but change its properties. According to Lindenberg, pinpointing every detail of how this happens is the first step towards keeping quantum dots shining in all devices. “For a long time there has been a desire to understand the kind of microscopic processes that determine the ultimate efficiencies of these materials,” he says.
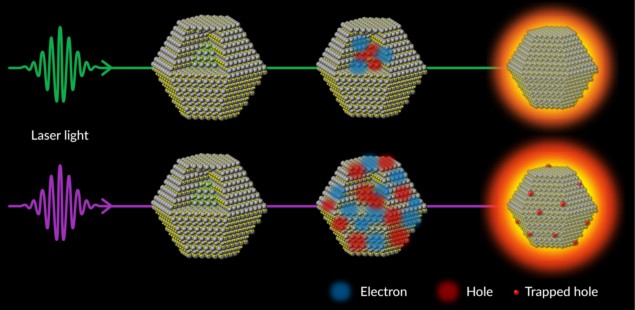
Because these microscopic processes take as little as as a trillionth of a second, they are extremely challenging to observe. The team behind the SLAC study, which appears in Nature Communications, got around this by exposing quantum dots to laser light, then firing very fast electrons at them almost immediately afterwards. By studying the way these electrons bounced off, the researchers inferred the details of the quantum dots’ atomic structure. Whenever an atom within the dot shifted, the electron ricochet pattern changed as well, providing clues as to how the shape of the dot was changing in real time.
When the laser frequency was low, the researchers found that whole dots wasted energy on warming up. With higher-frequency light, however, some of the electrons within the dots became excited enough to leave their usual places in the nanocrystal. This shifting of electrons left behind fast-moving, positively charged “holes” that travelled towards the surface of the nanocrystal. At that point, the holes got stuck, and the electric forces they exerted on the atoms around them caused the shape of the quantum dot surface to warp. In other words, the quantum dots were using the incident photon energy to contort themselves, rather than to emit light.
Caught red-handed
According to Gordana Dukovic, a physical chemist at the University of Colorado, Boulder, US, who was not involved in the study, scientists have previously speculated about the role of surface-trapped holes in quantum dot inefficiencies, but this is the first time anyone has seen these effects in action. “Trapped electrons and holes are notoriously difficult to observe,” she underscores. Her colleague Joel Eaves, who was also not involved in the SLAC study, agrees. “The fact that these researchers directly measured the lattice distortions that accompany hole trapping on the surface really is novel and impressive,” he says.
To better understand the troublesome electron-and-hole shuffle they observed, researchers also simulated the dots’ behaviour numerically. Agreement between simulations and experimental measurements strengthened the team’s hope that this new work could be used to improve future quantum dot technologies, notes Dmitri Talapin, a chemist at the University of Chicago, US and co-author on the study. Now that they have experimentally determined what kinds of photons lead to energetically costly distortions and hole trapping within the quantum dot, they can try to minimize these effects. “By tuning the colour of the light that you use to excite these nanocrystals, one could potentially control these processes that, in the end, are detrimental,” Lindenberg explains.

Quantum dot array could make ultralow-energy switches
Though such fine-tuning may not be needed for TV screens, it could make ultraviolet photodetectors more efficient or even lead to creation of new types of lasers built out of quantum dots. Talapin adds that though quantum dots are similar to traditional semiconductor technologies – a sort of smaller cousin to, for example, silicone-based devices – their size gives them different properties, making them promising candidates for future devices that might rely on absorbing energy from photons. “There is a strong consensus between the academic community and industrial communities that this class of materials sort of presents an interesting competitive platform as semiconductors 2.0,” he says. The experiment he and his collaborators conducted is a step towards making quantum dots an even stronger competitor in this field, he concludes.