
The way the eye moves in the moments after a head impact serves as a reliable proxy for the acceleration experienced by the brain, reports a research team in the US. The researchers observed the effect in a physical head phantom and a human volunteer, and say that the measurement could one day be made using “smart” contact lenses. Routine eye-motion measurements in athletes could allow sports-related traumatic brain injury (TBI) to be detected at the side-line (Physiol. Meas. 10.1088/1361-6579/ab78bd).
In American football, players selected in large part for their size and speed undergo multiple high-energy collisions per game. Perhaps unsurprisingly, mild TBI (concussion) is common, though players often do not realize it, or may deliberately downplay its symptoms to stay on the pitch. The most reliable ways to diagnose TBI are those that can only be administered away from the playing field: brain-imaging techniques like MRI or X-ray CT, or the detection of certain protein biomarkers in the blood.
Looking for a more accessible method that returns immediate results, some researchers have used helmet-based or head-mounted sensors, but with limited success. The reason that these whole-head measurements make a poor diagnostic tool is that the head kinematics do not necessarily reveal those of the brain inside. TBI occurs when the brain responds heterogeneously to linear and rotational accelerations of the skull. Shear forces that result from this heterogeneity are the cause of the brain trauma, but the details of the process are not fully understood.
“There are many known and unknown factors that may contribute to how the brain responds to a head impact,” says Yuan Meng of Auburn University. “One of the disappointing findings of the large-scale helmet-based telemetry system studies is that the same head kinematics can have a wide range of consequences, from no concussion at all to severe concussion.”
Meng, with colleagues at Auburn University and the University of Alabama at Birmingham, propose that the way the brain moves within the skull in response to a head impact might be reflected in the much more easily measured motion of the eye. The crucial interval is the 20 or so milliseconds immediately after the impact — before the ocular muscles impose stability, and when the eye moves purely according to the laws of dynamics.
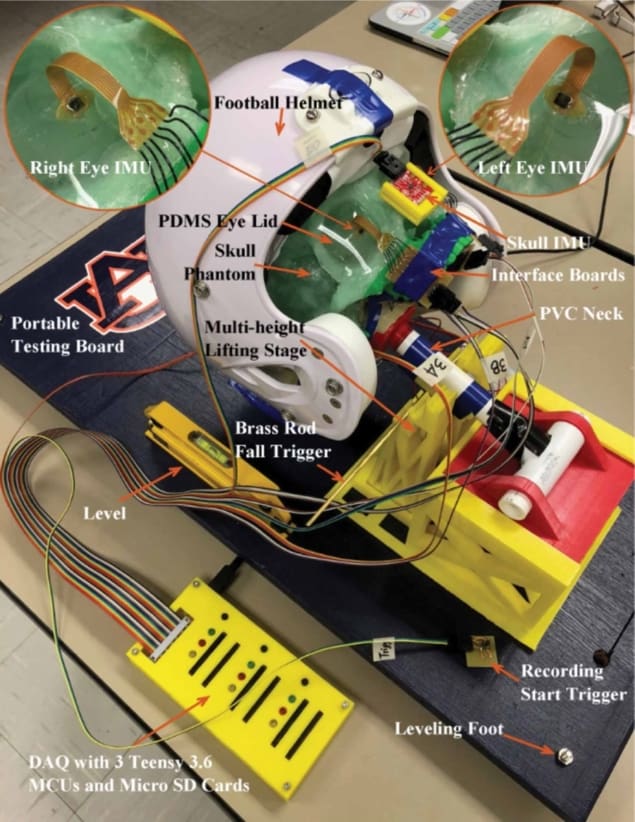
To test their proposal, the researchers constructed a human head phantom and subjected it to accelerations of the sort that could cause mild TBI in a living subject. They 3D-printed a skull in rigid plastic based on the dimensions of a real skull. They made the brain from a gelatin solution, while the eyeballs were composed of polydimethylsiloxane.
Microelectromechanical inertial measurement units (IMUs) placed on a diagonal line through the brain recorded how different regions responded to the acceleration of the phantom. Additional IMUs on the surfaces of the eyeballs (held underneath an artificial lower eyelid) measured the ocular response, while reference IMUs on the outside of the phantom measured the acceleration of the rigid skull.
The researchers placed the head phantom on a rotating vestibulo-ocular response (VOR) chair to induce rotational accelerations, and swung it on an inverted pendulum (like a falling tree) to induce linear accelerations. They found that the onset of acceleration in the brain occurred slightly later than that of the skull, and that the length of the delay depended on where the IMU was located relative to the rotation axis or direction of impact.
The phantom’s eyes experienced accelerations largely comparable to those recorded in the brain. In the rotational acceleration tests, despite the eyes being furthest from the axis of rotation, the delay before acceleration onset was in the middle of the range seen in the brain. Meng and colleagues attribute this to the effect of the small rigid eye socket.
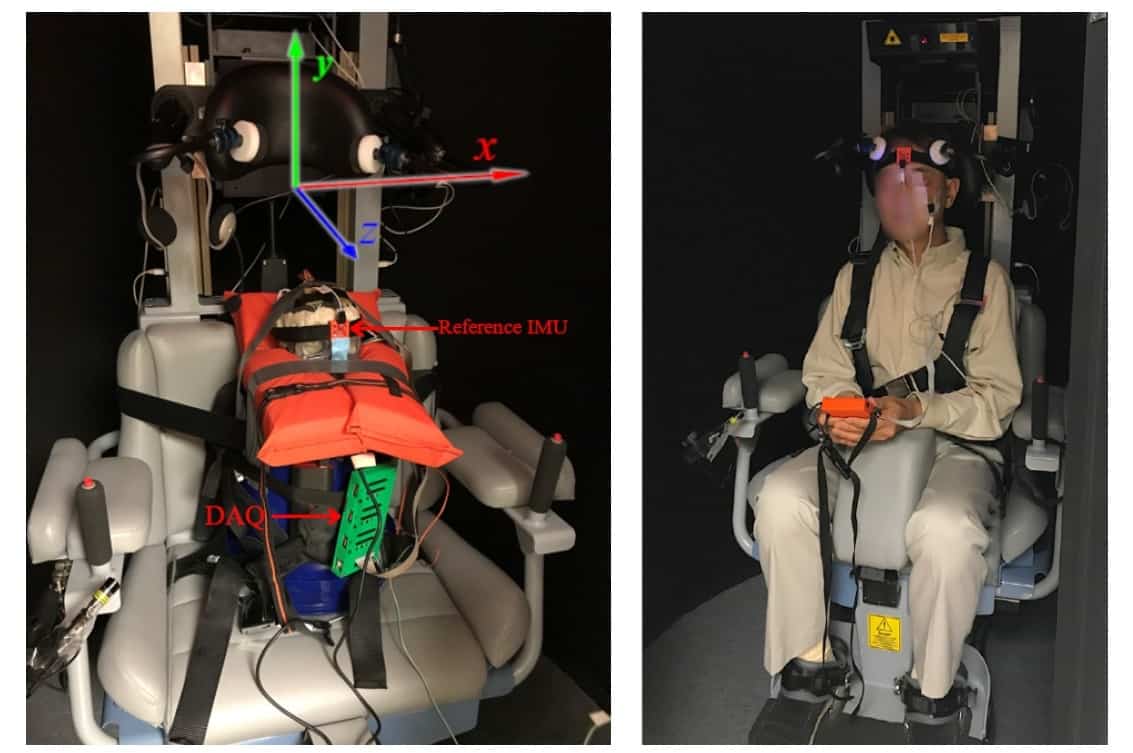
To demonstrate the feasibility of the technique in vivo, the researchers subjected a human volunteer to (non-injurious) rotational accelerations in the VOR chair, with an IMU placed beneath their lower eyelid. Although the researchers could not compare the measured eye movement to the motion of the subject’s brain, they confirmed that the eye’s passive response to acceleration was detectable before it was arrested by the ocular muscles.
The next step for the researchers is to miniaturize the IMUs and integrate them into a smart contact lens, which Meng says has already begun.
“We intend to test the prototype on more human volunteers and develop an entire real-time, on-field concussion monitoring system,” says Meng. “A wearable device based on this concept has a promising future in health monitoring in the context of sports, crashes and even on the battlefield.”



