
Improving the delivery of chemotherapy drugs to solid tumours while minimizing harmful side effects is key to optimizing cancer treatments. One promising approach lies in the use of thermosensitive liposomes (TSLs) that release encapsulated drugs upon heating, such as doxorubicin-loaded TSL (TSL-Dox), for example. TSL-Dox has shown encouraging outcomes in some patients, but its widespread clinical adoption is hindered by a lack of effective temperature control.
MRI-guided focused ultrasound (MRgFUS) could provide such control, enabling non-invasive heating of tissues to the hyperthermia regime of 41–43 °C. Previous preclinical studies of MRgFUS with TSL-Dox showed that raising tissue temperatures for tens of minutes to hours led to drug release and anti-tumour effects. But respiratory motion and cooling by blood make it difficult to maintain hyperthermia effectively. Instead, researchers in Canada propose a novel new approach: using MRgFUS to create fractionated ultrashort thermal exposures that release the encapsulated drug.
“It is very difficult to induce uniform temperature elevation and maintain it over long periods of time in a clinical setting, and even more difficult in an organ that moves with breathing, such as the liver,” explains lead author Kullervo Hynynen from Sunnybrook Research Institute and the University of Toronto. “Our aim was to use short ultrasound exposures that are short enough to be delivered during a patient breath-hold, thus eliminating the motion problem.”
Reducing each burst of heating to just 30 s also means that the temperature elevation is not highly dependent on the tissue’s blood perfusion rate, which is unknown and heterogenous throughout the tumour. As such, it should allow uniform thermal exposure over the entire tumour volume.
In vivo assessments
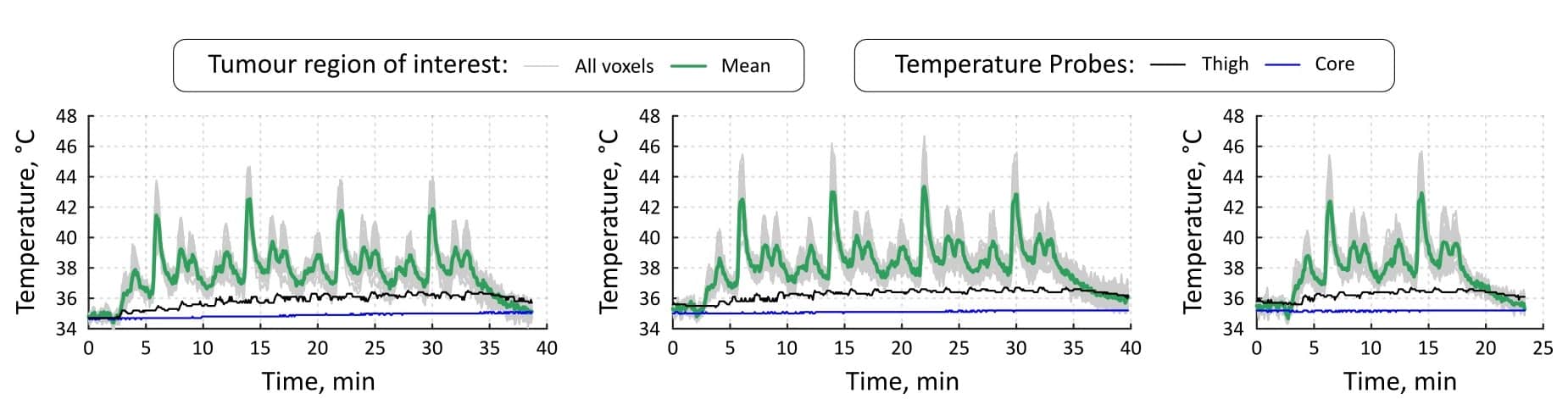
To determine whether the proposed ultrashort FUS exposures can release doxorubicin from TSL-Dox, the researchers first tested the approach using a dorsal skinfold chamber on a mouse with implanted tumour cells. After injecting TSL-Dox, they exposed the tumours to ten 30 s FUS thermal exposures, using thermocouple-based temperature-based feedback to heat the tissue to 41°, 42°, 43° or 45°C.
The experiment showed that the ultrashort thermal exposures could effectively release the doxorubicin and that the drug was subsequently taken up by tumour cells. Heating to 42 °C appeared to give the most consistent increases in drug delivery to the mouse tumours. Thus the team chose this temperature to evaluate the therapeutic efficacy of fractionated ultrashort MRgFUS heating with TSL-Dox in rabbit thigh tumours.
For the studies on rabbits, the researchers employed a custom MRI-compatible FUS system to deliver short-duration heating, with MR thermometry providing real-time temperature feedback during the treatment. They used MRI to identify the tumours and define discrete regions-of-interest (ROIs) to encompass the tumour. After administering either TSL-Dox or a control dextrose, each ROI was heated 10 times, to 42 °C for 30 s. The FUS was delivered via a single-focus transducer translated through a series of discrete locations to achieve tumour coverage.
Examining drug delivery to excised bilateral tumours (one heated, one not heated) demonstrated that the level of doxorubicin fluorescence was markedly increased in the heated versus the unheated tumour sections – indicating the release of drug, which does not fluoresce when in liposomal form.
The researchers also evaluated the anti-tumour efficacy of the treatment. They saw a significant improvement in survival for rabbits receiving MRgFUS plus TSL-Dox compared with animals receiving MRgFUS alone or TSL-Dox alone. Of six rabbits treated with MRgFUS plus TSL-Dox, three survived to 120 days following treatment, four showed complete tumour destruction and only one reached the tumour size-related endpoint.

Focused ultrasound releases cancer drugs on target
Follow-up MR images showed a marked decrease in tumour size in rabbits treated with MRgFUS plus TSL-Dox. Conversely, all 11 rabbits that received either MRgFUS or TSL-Dox alone showed tumour growth, and all reached the tumour size-related endpoint within 28 days.
One week after treatment, tumours in the group that received MRgFUS alone were significantly larger than in the group receiving MRgFUS plus TSL-Dox. Two weeks after treatment, rabbits in both the MRgFUS alone and the TSL-Dox alone groups had significantly larger tumours than those treated with MRgFUS plus TSL-Dox.
The researchers conclude that these results have considerable implications for the widespread clinical adoption of thermosensitive drugs in oncology. Hynynen tells Physics World that the team is now working on translating the method for clinical testing.
The findings are published in Science Advances.



