
The ability of focused ultrasound to heat tissue enables a range of non-invasive therapies, such as tumour ablation, for example, or relief of essential tremor. Another application under investigation is the use of ultrasound to induce mild hyperthermia (heating by no more than 6°C) and trigger drug release from thermosensitive liposomal carriers, enabling targeted drug delivery to tumours.
There are, however, challenges in translating ultrasound-induced drug release from small-animal studies to clinical use, including identification of a suitable technique for monitoring the heating process. MRI thermometry can measure temperature in real time, but its high costs limit widespread application. Implanted temperature sensors, meanwhile, are necessarily invasive and may increase risk to the patient, as well as limiting which patients can be monitored.
Now, a team from the University of Oxford has investigated the use of computational planning models to determine the focused ultrasound parameters required to release drugs from liposomes, without the need for real-time thermometry. Such an approach could make ultrasound-mediated targeted drug delivery more widely accessible and simpler to administer than techniques that employ MRI guidance (Radiology 10.1148/radiol.2018181445).
“A key objective in developing the planning model was to not only validate the safety and feasibility of non-invasively triggered drug delivery in oncology, but to do so in a way that would enable large-scale adoption and deployment of the technique if successful,” explains first author Michael Gray. “We hypothesized that neither expensive nor invasive thermometry would be necessary for mild hyperthermia-based treatments employing non-invasive ultrasound for targeted heating.”
Patient-specific models
The study, part of the TARDOX trial, included 10 participants with liver tumours who were treated using focused ultrasound to release the cancer drug doxorubicin from liposomes. For the first six patients (group 1), the researchers used an implanted sensor to monitor temperature during ultrasonic heating of target tumours. They treated the other four patients without real-time thermometry, in a step towards fully non-invasive therapy.

Gray and colleagues developed a model to create focused ultrasound treatment plans, using a combination of participant data and finite element calculations. For each patient, the model recommends treatment parameters and predicts the resulting temperature fields.
“Patient images were used to create a combined acoustic/thermal anatomical model of the patient and target tumour,” explains Gray. “Based on segmentation of CT and MRI data, each tissue type and tissue layer was assigned specific acoustic and thermal properties to enable predictive modelling of acoustic propagation and ultrasound-mediated hyperthermia.”
The calculated treatment parameters showed that the prescribed power scaled approximately with target depth. For seven patients in whom model predictions were available at the time of treatment, the differences between predicted and implemented powers (mean of 3.5 W) were not clinically significant relative to the power used (mean of 64 W). These results indicate that the model consistently provides settings that are safe and agree with those chosen in the presence of thermometry.
The team also retrospectively created models for the first three participants, who were treated before model availability. The largest prediction discrepancy was seen for a patient with a target nearly 5 cm deeper than any others. On the basis of thermometry, this treatment was substantially underpowered. The researchers note that using model-predicted settings (had they been available) should have improved target heating.
For the three group 1 participants with treatment volumes of 52 cm3 or less (about the size of a golf ball), measured treatment-averaged temperatures were within 0.1–0.3°C of the model predictions. For participants with larger tumours, the prediction was 1.4–1.7°C below the measured value, suggesting that the small sensor used was not a reliable indicator of the median temperature of larger targets.
Treatment response
To assess therapeutic response, the team imaged all participants before and after treatment using contrast-enhanced MRI and CT, and FDG-PET/CT. In the first patient, PET revealed a 36.4% reduction in total lesion glycolysis of the target tumour, whereas there was no substantial response in a similarly sized tumour that received drug but no focused ultrasound. In six of the 10 participants, partial responses were seen after just one treatment cycle.
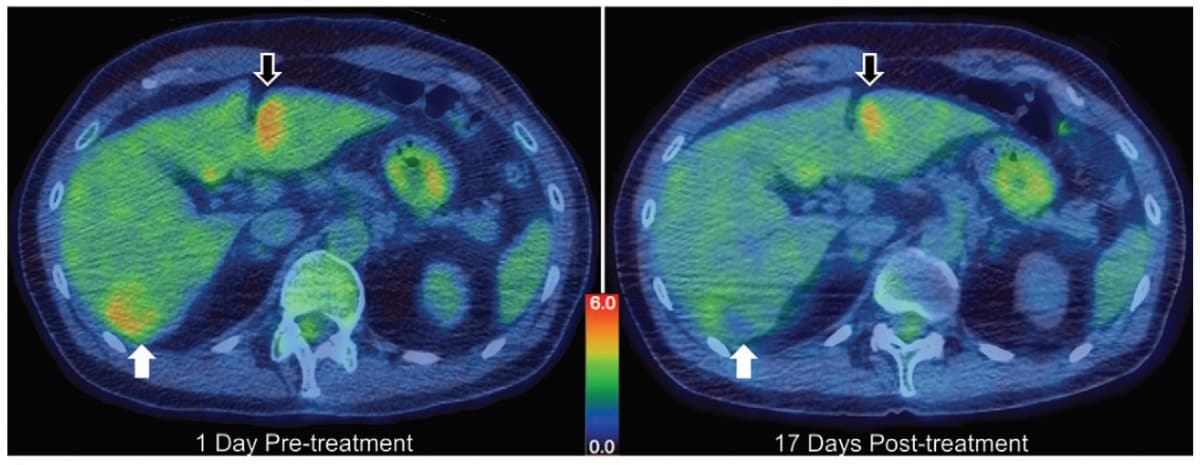
Histologic and MRI data showed no evidence of thermal tissue ablation, confirming the safety of this procedure. There were no skin burns, off-target tissue damage, or other clinically significant adverse effects related to focused ultrasound. The researchers also found that the model-prescribed treatments resulted in similar levels of enhanced drug delivery with or without real-time thermometry.
The team concluded that the study supports the feasibility and safety of using planning models to define treatment parameters for targeted hyperthermic drug delivery to liver tumours without real-time thermometry.
“We are currently working on several additional clinical applications for the techniques developed in TARDOX, and hope to begin another Phase 1 trial in another indication in the next 12 months,” Gray tells Physics World. “We are also exploring ultrasound-enhanced drug delivery using cavitational rather than thermal ultrasound mechanisms and will be starting a first-in-man study of this approach over the course of 2020.”



