A new standard for pressure measurement that does not rely on artefacts such as mechanical pistons or columns of mercury has been developed by researchers in Germany. The method, which draws instead on first-principles calculations and sensitive measurements of the electrical properties of helium gas, is accurate to within 5 parts per million at pressures of up to 7 MPa and could eventually replace pressure standards based on physical objects.
In the mid-1600s, scientists such as Evangelista Torricelli and Christiaan Huygens began using open-ended columns and tubes of mercury to measure the pressure exerted by a gas relative to atmospheric pressure. Systems of this type are still used as pressure standards, but in recent decades metrologists have worked to develop alternatives that eliminate the need for toxic mercury. The new pressure standard grew out of one such effort, led by Christof Gaiser and colleagues at the Physikalisch-Technische Budesanstalt Institut (PTB) in Berlin.
The PTB team’s first step was to replace mercury columns with precision-engineered pistons. The pressure below a piston can be calculated in a straightforward way, by multiplying the surface area of the piston by the mass of the load. The difficulty, Gaiser explains, is that both the surface area of the piston and the gap between the piston and the surrounding cylinder must be measured to a very high degree of accuracy. Moreover, since the pistons are physical objects, each one is slightly different. “The piston gauges we have at PTB with an uncertainty of one part per million are artefacts,” Gaiser says. “They are all unique, and they must be characterized very accurately.”
An independent method
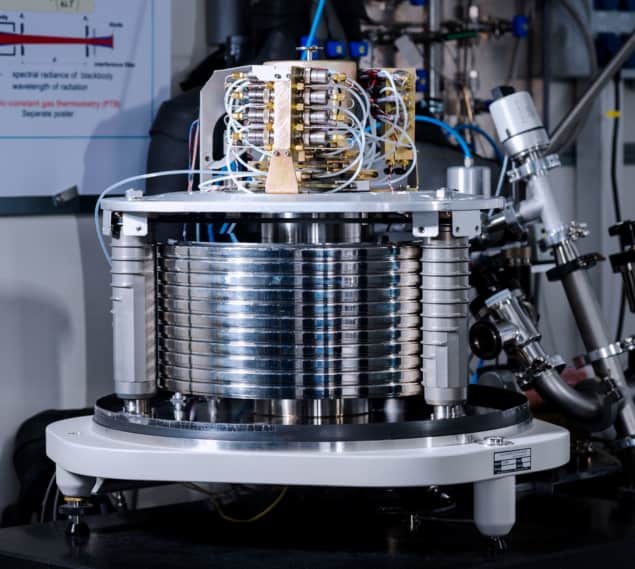
To eliminate this disadvantage, and to check the accuracy of their mechanical piston gauges, Gaiser and colleagues developed an alternative gas-pressure standard based on a technique known as dielectric constant gas thermometry (DCGT). This method, which was invented in the early 1980s and refined at PTB as part of an international effort to fix the value of the Boltzmann constant (and thereby redefine the kelvin unit of temperature), involves measuring how electrical capacitance changes when the space between a capacitor’s electrodes is filled with a pressurized gas. The change in capacitance is related to the gas’ dielectric constant, which depends in turn on its density. Once you know the density, Gaiser says, it is straightforward to calculate temperature using a modified version of the ideal gas law.
The PTB team’s latest result offers a new twist on DCGT. Instead of using the change in capacitance to calculate temperature, they used it to derive pressure, taking advantage of the now-fixed definition of temperature and theoretical calculations of two quantities: the electrical polarizability of the gas and the strength of interactions between gaseous atoms.
In helium, such calculations can be performed from first principles. Gaiser notes that they have a long history, with the first values for helium’s electrical polarizability being derived in the 1920s and 1930s. More recently, the kelvin-redefinition project spurred major advances in the accuracy of these calculations, with several research groups independently developing better methods of performing them. These improvements, together with the PTB group’s own work in the laboratory, made it possible to develop the new capacitance-based standard.
‘Probably the world’s best pressure measurements’
“[The PTB researchers] had to do (probably) the world’s best pressure measurements when determining the Boltzmann constant by DCGT, so I am not surprised you can do the reverse and use a similar approach to measure pressure accurately,” says Graham Machin, a fellow at the National Physical Laboratory in Teddington, UK.
Machin, who led the UK’s contribution to redefining the kelvin but was not involved in the present work, says that the PTB team’s method could form the basis of a future non-mechanical pressure standard. Although he cautions that mechanical standards will not be replaced overnight, “in the longer term, when older standards reach the end of their life, this would be a serious alternative.”
James Schmidt, a physicist and pressure expert at the US National Institute of Standards and Technology (NIST), concurs. “In the same way that the kelvin has been replaced by a definition of the Boltzmann constant, the pressure scale could be replaced by the density of a well-characterized gas and electronic measurements of either the dielectric permittivity or refractive index of that gas,” he says. “While the re-definitions and new techniques may not immediately replace operations on the factory floor, they are important for a few of the highest-level national standards institutions, such as PTB and NIST.”
Gaiser agrees that the PTB method will need further work before it is widely adopted. “It’s a quite huge experiment and you have to take into account that you need a very pure gas and good temperature stability,” he says. Pushing the method beyond its current 7 MPa limit will also require further advances in the theory of helium gas at very high pressures, he adds.
The team report their work in Nature Physics.