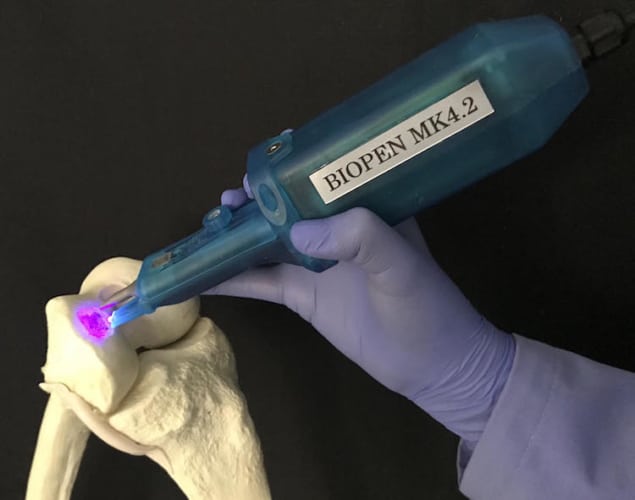
A handheld “biopen” capable of 3D printing cartilage tissue could for the first time be used during surgery to treat cartilage injuries and osteoarthritus. The extrusion-based device, which prints live stem cells embedded in a hydrogel material, produces constructs that look and behave just like natural articular tissue (Biofabrication 10 045006).
“This is in stark contrast to conventional reparative cartilage made of fibrocartilage, which is very different in structure to physiological cartilage, inferior in quality and not durable,” say the researchers, a multidisciplinary team that includes surgeons, biologists, physicists and engineers. “Our technique and the scaffolds we are able to produce provide much hope for treating patients suffering from cartilage injuries and osteoarthritis.”
Cartilage is a highly specialized tissue that has so far proved difficult to replicate using conventional tissue engineering techniques. Its special mechanical properties arise from the fact that it contains only a few cells, does not contain blood vessels, and has a distinct 3D collagen fibre structure comprising aggregated proteoglycans in an organized matrix.
While some success has been reported for surgical treatments that exploit engineered cartilage tissue, existing procedures require two separate operations: one to remove the damaged tissue, and another to replace the tissue once it is repaired. What’s more, surgeons report a high failure rate – partly because pre-fabricated scaffolds might not perfectly match the defect, and partly because the implanted tissue is not similar enough to natural cartilage to survive for long inside the body.
Researchers believe that treatments using human stem cells could offer a solution, but few studies have been reported to date. In this new work, reported in the journal Biofabrication, scientists from the University of Melbourne, the University of Wollongong and the St Vincent’s Hospital of Melbourne provide in vitro evidence that their new biopen could enable human stem cells to be 3D printed inside the operating theatre. The ultimate aim is to enable surgeons to “sculpt” patient-specific structures in real time, creating scaffolds that perfectly match the geometry of the defect and achieve the best possible contact between the bioscaffold and the host tissue.
The team tested their approach with human-derived mesenchymal stem cells (hADSCs) that had been harvested from the infra-patellar fat pad of donor patients with osteoarthritis. Once the stem cells had been embedded in a hydrogel material, the resulting bioink could be extruded through the biopen to create a cell-laden bioscaffold.
One key feature of the new biopen is a specially designed nozzle that allows cells and biomaterials contained in separate cartridges to be extruded co-axially. The inner core of the extruded material contains live stem cells, while the outer shell contains cross-linked material that provides rigidity to the printed construct.
In this study the researchers used the biopen to print 3D scaffolds laden with stem cells, which were then cultured in vitro for eight weeks in the presence of cartilage-forming media. “Thanks to a series of sophisticated histological, molecular and imaging techniques, we confirmed that the tissue that developed within the constructs forms, appears, and behaves like articular cartilage,” say the researchers. “This material is expected to be as durable as real cartilage.”

Biopen speeds up stem-cell repair
This detailed in vitro analysis is a crucial step towards full-scale clinical trials. The group has already shown that the device could be used in a real-world setting through preliminary in vivo experiments in a large animal model, and more extensive in vivo testing will be needed before the biopen can be tested on humans.
At the same time, the researchers are investigating whether the same technology could be applied to other organs and clinical scenarios, while also looking at ways to speed up and streamline the processes needed during surgery.
“We are refining our methodology to upscale the production of the inks used in the device, and to fine-tune how to harvest and prepare the mesenchymal stem cells,” they say. “We are also aiming to develop a protocol that will enable one-step harvesting, preparation and delivery of the cells during surgery.”
- Read our special collection “Frontiers in biofabrication” to learn more about the latest advances in tissue engineering. This article is one of a series of reports highlighting high-impact research published in Biofabrication.