Astronauts spending time in the harsh environment of space often experience damaging effects on their health, including a deterioration in heart function. Indeed, the landmark NASA Twins Study found that an astronaut who spent a year on the International Space Station (ISS) had significantly increased cardiac output and reduced arterial pressure compared with his identical twin who remained on Earth. And with missions planned to Mars and beyond, there’s an urgent need to understand how long-duration spaceflight affects the cardiovascular system.
With this aim, a research team headed up at Johns Hopkins University has sent a heart-on-a-chip platform to the International Space Station and investigated the impact of 30 days in space on the cardiac cells within. The findings, reported in the Proceedings of the National Academy of Sciences, could also shed light on the changes in heart structure and function that occur naturally due to ageing.
“I began cardiac research after my own father died of heart disease when I was a senior college student, and my main motivation for studying the effects of spaceflight on cardiac cells stemmed from the striking resemblance between cardiac deterioration in microgravity and the ageing process on Earth,” project leader Deok-Ho Kim tells Physics World. “The ability to counteract the impacts of microgravity on cardiac function will be essential for prolonged duration human spaceflights, and may lead to therapies for ageing hearts on Earth.”
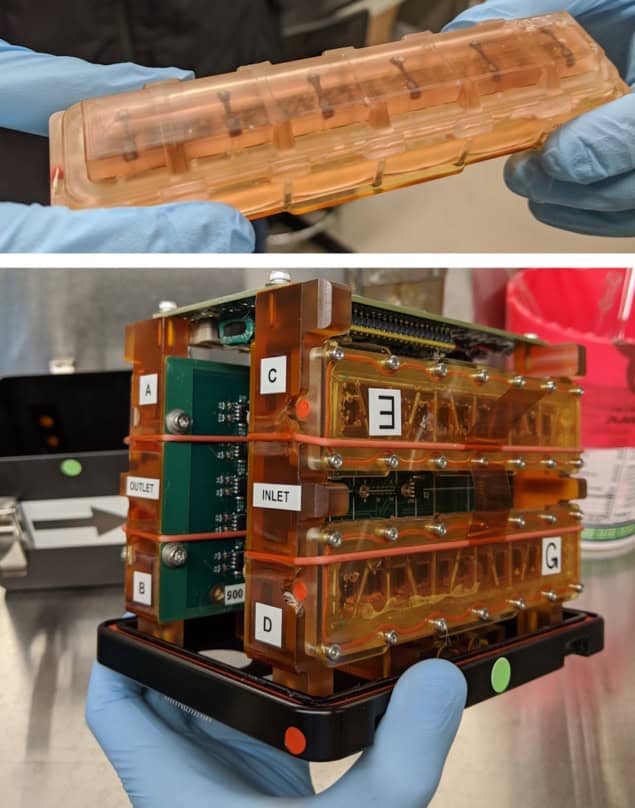
The heart-on-a-chip platform is based on engineered heart tissues (EHTs), in which heart muscle cells (cardiomyocytes) derived from human-induced pluripotent stem cells are cultured within a hydrogel scaffold. The key advantage of this design over previous studies using 2D cultured cells is its ability to more accurately replicate human cardiac muscle tissue.
“Cells cultured on traditional 2D petri dishes do not behave as they would in the body, whereas our platform provides a physiologically relevant 3D environment that mimics in vivo conditions,” Kim explains.
Inside the platform, the EHTs are mounted between two posts, one of which is flexible and contains a small magnet that moves as the tissue contracts. Small magnetic sensors measure the changes in magnetic flux to determine tissue contraction in real time.
Designed for space
To allow culture of the cardiac cells in microgravity, Kim’s team – primarily postdoctoral fellow Jonathan Tsui – developed custom sealed tissue chambers containing six EHTs. These chambers, along with the magnetic sensors and associated electronics, were housed within a compact plate habitat that required minimal handling to maintain cell viability. “The platform was designed to be easily maintained by astronauts aboard the ISS, an important consideration as crew time is a precious resource,” says Kim.
The tissue chambers were carefully transported by Tsui to the Kennedy Space Center, then launched to the ISS aboard the SpaceX CRS-20 mission in March 2020. The researchers then monitored the function of the cardiac tissues for 30 days in microgravity, using the sensors to automatically detect magnet motion as the cells beat. The raw data were transmitted down from the ISS and converted into force and frequency measurements that provided insight into the contraction strength and beating patterns, respectively.
Once the samples were back on Earth, the researchers examined the cardiac tissues during a nine-day recovery period. They compared their findings with results from an identical set of EHTs cultured on Earth for the same duration.
Cardiac impact
After 12 days on the ISS, the EHTs exhibited a significant decrease in contraction strength compared with both baseline values and the control EHTs on Earth. This reduction persisted throughout the experiment and during the recovery period on Earth. The cardiac tissues also exhibited increased incidences of arrhythmia (irregular heart rhythm) whilst on the ISS, although this resolved once back on Earth.
At the end of the experiment (day 39), Kim and colleagues examined the cardiac tissue using transmission electron microscopy. They found that spaceflight caused sarcomeres (protein bundles that help muscle cells contract) to become shorter and more disordered – a marker of human heart disease. The changes did not resolve after return to Earth and may be why the cardiac tissues did not regain contraction strength in the recovery period. The team also observed mitochondrial damage in the cells, including fragmentation, swelling and abnormal structural changes.
To further assess the impact of prolonged microgravity, the researchers performed RNA sequencing on the returned tissue samples. They observed up-regulation of genes associated with metabolic disorders, heart failure, oxidative stress and inflammation, as well as down-regulation of genes related to contractility and calcium signalling. Finally, they used in silico modelling to determine that spaceflight-induced oxidative stress and mitochondrial dysfunction were key to the tissue damage and cardiac dysfunction seen in space-flown EHTs.
Study in astronauts could improve health in space and on Earth
“By conducting a detailed investigation into cellular changes under real microgravity conditions, we aimed to uncover the mechanisms behind these alterations, potentially leading to therapies that could benefit both astronauts and the ageing population,” says Kim.
Last year, the researchers sent a second batch of EHTs to the ISS to screen drugs that may protect against the effects of low gravity. They are currently analysing the data from these studies. “These results will help us refine the effectiveness of promising drug therapies for our upcoming third mission,” says Kim.